|
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylonitrile
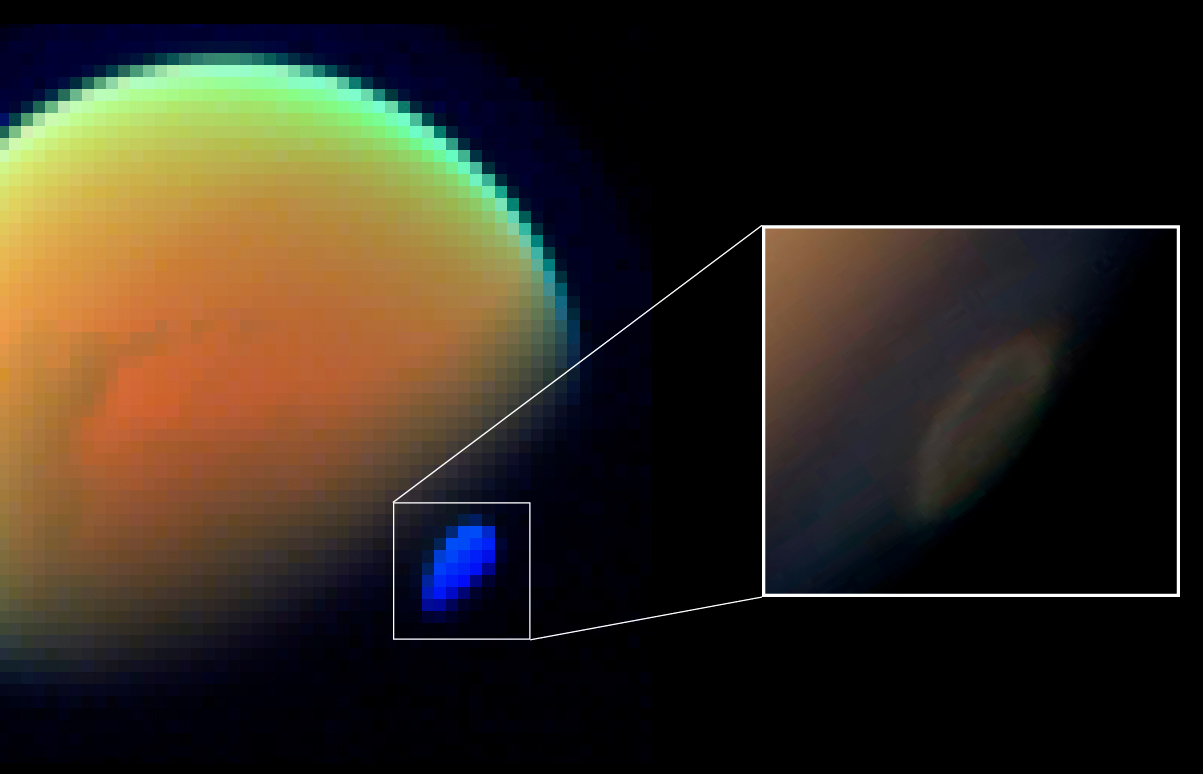
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular structure, it consists of a vinyl group () linked to a nitrile (). It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses. Acrylonitrile was first synthesized by the French chemist Charles Moureu (1863–1929) in 1893. Occurrence Acrylonitrile is not naturally formed on Earth. It has been detected at the sub-ppm level at industrial sites. It persists in the air for up to a week. It decomposes by reacting with oxygen and hydroxyl radical to form formyl cyanide and formaldehyde. Acrylonitrile is harmful to aquatic life. Acrylonitrile has been detected in the atmosphere of Titan, a moon of Saturn. Computer simulations suggest that on Titan conditions exist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionitrile
Propionitrile, also known as ethyl cyanide and propanenitrile, is an organic compound with the formula CH3CH2CN. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid. It is used as a solvent and a precursor to other organic compounds. Production The main industrial route to this nitrile is the hydrogenation of acrylonitrile. It is also prepared by the ammoxidation of propanol (propionaldehyde can also be used instead):Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. :CH3CH2CH2OH + O2 + NH3 → CH3CH2CN + 3 H2O Propionitrile is a byproduct of the electrodimerisation of acrylonitrile to adiponitrile. In the laboratory propanenitrile can also be produced by the dehydration of propionamide, by catalytic reduction of acrylonitrile, or by distilling ethyl sulfate and potassium cyanide. Applications Propionitrile is a solvent simila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. Structure and general properties Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide. Hydrogen cyanide is weakly acidic with a p''K''a of 9.2. It partially ionizes in water solution to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called ''hydrocyanic acid''. The salts of the cyanide ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons. Inorganic compounds containing the group are not called nitriles, but cyanides instead. Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic. Structure and basic properties The N−C−C geometry is linear in nitriles, reflecting the sp hybridization of the triply bonded carbon. The C−N distance is short at 1.16 Å, consistent with a triple bond. Nitriles a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. Structure and general properties Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide. Hydrogen cyanide is weakly acidic with a p''K''a of 9.2. It partially ionizes in water solution to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called ''hydrocyanic acid''. The salts of the cyanide ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone Cyanohydrin
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic. Preparation In the laboratory, this compound may be prepared by treating sodium cyanide with acetone, followed by acidification: : Considering the high toxicity of acetone cyanohydrin, a lab scale production has been developed using a microreactor-scale flow chemistry to avoid needing to manufacture and store large quantities of the reagent. Alternatively, a simplified procedure involves the action of sodium or potassium cyanide on the sodium bisulfite adduct of acetone prepared ''in situ''. This gives a less pure product, one that is nonetheless suitable for most syntheses. Reactions Acetone cyanohydrin is an intermediate en route to methyl methacrylate. Treat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pivalonitrile
Pivalonitrile is a nitrile with the semi-structural formula (CH3)3CCN, abbreviated ''t''-BuCN. This aliphatic organic compound is a clear, colourless liquid that is used as a solvent and as a labile ligand in coordination chemistry. Pivalonitrile is isomeric with ''tert''-butyl isocyanide but the two compounds do not exist in chemical equilibrium, unlike its silicon analog trimethylsilyl cyanide Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen .... References 5 Tert-butyl compounds {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminopropionitrile
Aminopropionitrile, also known as β-aminopropionitrile (BAPN), is an organic compound with both amine and nitrile functional groups. It is a colourless liquid. The compound occurs naturally and is of interest in the biomedical community. Biochemical and medical occurrence BAPN is the toxic constituent of peas from Lathyrus plants, e.g., lathyrus odoratus. Lathyrism, a disease known for centuries, encompasses 2 distinct entities: a disorder of the nervous system (neurolathyrism) leading to limb paralysis, and a disorder of connective tissue, causing either bone deformity (osteolathyrism) or aortic aneurisms (angiolathyrim). BAPN causes osteolathyrism and angiolathyrism when ingested in large quantities." It can cause osteolathyrism, neurolathyrism, and/or angiolathyrism. It is an antirheumatic agent in veterinary medicine. It has attracted interest as an anticancer agent. Production Aminopropionitrile is prepared by the reaction of ammonia with acrylonitrile.Karsten Eller, Erha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malononitrile
Malononitrile is an organic compound nitrile with the formula . It is a colorless or white solid. It can be prepared by dehydration of cyanoacetamide. Malononitrile is relatively acidic, with a p''K''a of 11 in water. This allows it to be used in the Knoevenagel condensation, for example in the preparation of CS gas: Malononitrile is a suitable starting material for the Gewald reaction, where the nitrile condenses with a ketone or aldehyde in the presence of elemental sulfur and a base to produce a 2-aminothiophene. See also * Malonic acid * Diethyl malonate Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds su ... References External links WebBook page for C3H2N2 {{Authority control Alkanedinitriles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen
Cyanogen is the chemical compound with the formula ( C N)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C‒ C≡N, although other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr) (but see also ''Cyano radical''.) Cyanogen is the anhydride of oxamide: :H2NC(O)C(O)NH2 → NCCN + 2 H2O although oxamide is manufactured from cyanogen by hydrolysis: :NCCN + 2 H2O → H2NC(O)C(O)NH2 Preparation Cyanogen is typically generated from cyanide compounds. One laboratory method entails thermal decomposition of mercuric cyanide: :2 Hg(CN)2 → (CN)2 + Hg2(CN)2 Alternatively, one can combine solutions of copper(II) salts (such as copper(II) sulfate) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolonitrile
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin and it is derived from formaldehyde. It is a colourless liquid that dissolves in water and ether. Because glycolonitrile decomposes readily into formaldehyde and hydrogen cyanide, it is listed as an extremely hazardous substance. In January 2019, astronomers reported the detection of glycolonitrile, another possible building block of life among other such molecules, in outer space. Synthesis and reactions Glycolonitrile is produced by reacting formaldehyde with hydrogen cyanide under acidic conditions. This reaction is catalysed by base..Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. Glycolonitrile polymerizes under alkaline conditions above pH 7.0. As the product of polymerization is an amine with a basic char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoacetonitrile
Aminoacetonitrile is the organic compound with the formula NCCH2NH2. The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is usually encountered as the chloride and bisulfate salts of the ammonium derivative, i.e., CCH2NH3sup>+Cl− and CCH2NH3sup>+HSO4−. Production and applications Industrially aminoacetonitrile is produced from glycolonitrile by reaction with ammonia: :HOCH2CN + NH3 → H2NCH2CN + H2O The aminoacetonitrile can be hydrolysed to give glycine: Being bifunctional, it is useful in the synthesis of diverse nitrogen-containing heterocycles. Aminoacetonitrile derivatives are useful antihelmintics. They act as nematode specific ACh agonists causing a spastic paralysis and rapid expulsion from the host. Occurrence in the interstellar medium Using radio astronomy, aminoacetonitrile was discovered in the Large Molecule Heimat, a giant gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |