|
AMPA Receptor
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic receptor, ionotropic transmembrane receptor for glutamate (iGluR) that mediates fast synapse, synaptic transmission in the central nervous system (CNS). It has been traditionally classified as a non-NMDA_receptor, NMDA-type receptor, along with the kainate receptor. Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualic acid, quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The ''GRIA2''-encoded AMPA receptor ligand binding core (GluA2 LBD) was the first glutamate receptor ion channel domain to be protein crystal, crystallized. Structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AMPA Receptor
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic receptor, ionotropic transmembrane receptor for glutamate (iGluR) that mediates fast synapse, synaptic transmission in the central nervous system (CNS). It has been traditionally classified as a non-NMDA_receptor, NMDA-type receptor, along with the kainate receptor. Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualic acid, quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The ''GRIA2''-encoded AMPA receptor ligand binding core (GluA2 LBD) was the first glutamate receptor ion channel domain to be protein crystal, crystallized. Structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramer Protein
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as Sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be determined fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP : ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CACNG2
Calcium channel, voltage-dependent, gamma subunit 2, also known as CACNG2 or stargazin is a protein that in humans is encoded by the ''CACNG2'' gene. Function L-type calcium channels are composed of five subunits. The protein encoded by this gene represents one of these subunits, gamma, and is one of several gamma subunit proteins. It is an integral membrane protein that is thought to stabilize the calcium channel in an inactive (closed) state. This protein is similar to the mouse stargazin protein, mutations in which having been associated with absence seizures, also known as petit-mal or spike-wave seizures. This gene is a member of the neuronal calcium channel gamma subunit gene subfamily of the PMP-22/EMP/MP20 family. Stargazin is involved in the transportation of AMPA receptors to the synaptic membrane, and the regulation of their receptor rate constants — via its extracellular domain — once it is there. As it is highly expressed throughout the cerebral cortex, it is l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PSD-95
PSD-95 (postsynaptic density protein 95) also known as SAP-90 (synapse-associated protein 90) is a protein that in humans is encoded by the ''DLG4'' (discs large homolog 4) gene. PSD-95 is a member of the membrane-associated guanylate kinase The membrane-associated guanylate kinases (MAGUK) are a superfamily of proteins. The MAGUKs are defined by their inclusion of PDZ, SH3 and GUK domains, although many of them also contain regions homologous of CaMKII, WW and L27 domains. The G ... (MAGUK) family. With DLG2, PSD-93 it is recruited into the same NMDA receptor and potassium channel clusters. These two MAGUK proteins may interact at postsynaptic sites to form a multimeric scaffold for the Protein complex, clustering of receptors, ion channels, and associated signaling proteins. PSD-95 is the best studied member of the MAGUK-family of PDZ domain-containing proteins. Like all MAGUK-family proteins, its basic structure includes three PDZ domains, an SH3 domain, and a guanylate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
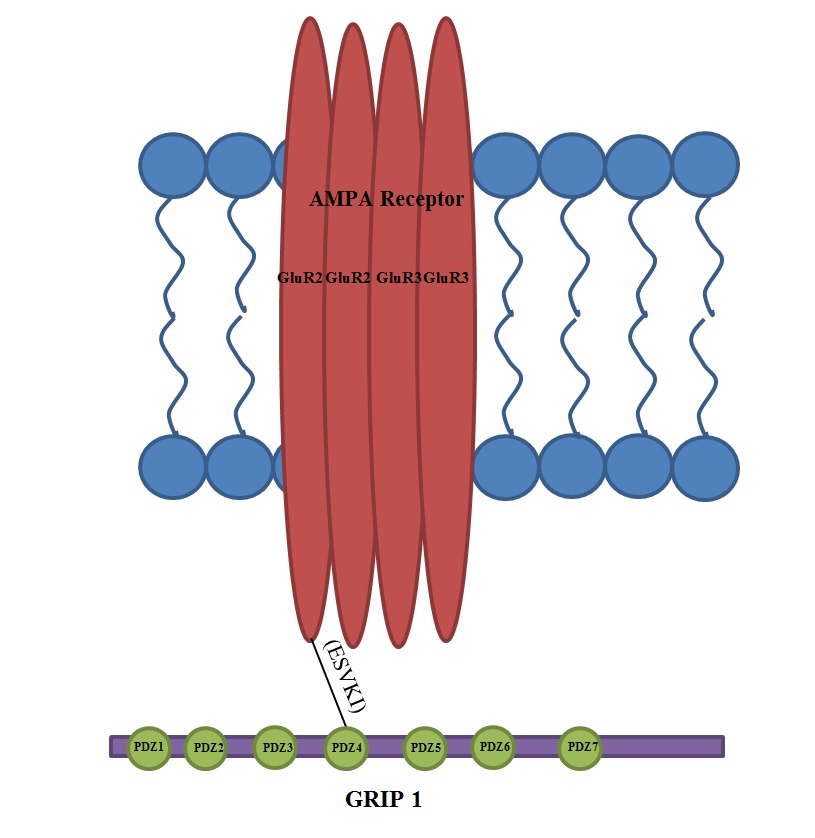
Glutamate Receptor-interacting Protein (GRIP)
Glutamate receptor-interacting protein (GRIP) refers to either a family of proteins that bind to the glutamate receptor or specifically to the GRIP1 protein within this family. Proteins in the glutamate receptor-interacting protein (GRIP) family have been shown to interact with GluR2, a common subunit in the AMPA receptor. This subunit also interacts with other proteins such as protein interacting with C-kinase1 (PICK1) and N-ethylmaleimide-sensitive fusion protein (NSF). Studies have begun to elucidate its function; however, much is still to be learned about these proteins. Discovery and history of GRIP 1 The discovery of the Glutamate Receptor Interacting Protein (GRIP-1) came as a result of the observation that Glutamate Receptors, such as the NMDA receptor, cluster at synapses. Shortly after this observation, researchers identified a region on the C-terminal region of NMDA receptors called the tSXV motif that has the ability to bind to the PDZ domain of the PSD-95 protein. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PICK1
Protein Interacting with C Kinase - 1 is a protein that in humans is encoded by the ''PICK1'' gene. Function The protein encoded by this gene contains a PDZ domain, through which it interacts with protein kinase C, alpha (PRKCA). This protein may function as an adaptor that binds to and organizes the subcellular localization of a variety of membrane proteins. It has been shown to interact with multiple glutamate receptor subtypes, monoamine plasma membrane transporters, as well as non-voltage gated sodium channels, and may target PRKCA to these membrane proteins and thus regulate their distribution and function. This protein has also been found to act as an anchoring protein that specifically targets PRKCA to mitochondria in a ligand-specific manner. Three transcript variants encoding the same protein have been found for this gene. Interactions PICK1 has been shown to interact with: * ACCN2, * BNC1, * Dopamine transporter, * GRIA2, * GRIA3, * GRIA4, * GRIK1, * GRIK2, * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SAP97
Discs large homolog 1 (DLG1), also known as synapse-associated protein 97 or SAP97, is a scaffold protein that in humans is encoded by the ''SAP97'' gene. SAP97 is a mammalian MAGUK-family member protein that is similar to the Drosophila protein Dlg1 (the protein is alternatively referred to as hDlg1, and the human gene is DLG1). SAP97 is expressed throughout the body in epithelial cells. In the brain it is involved in the trafficking of ionotropic receptors from the endoplasmic reticulum to the plasma membrane, and may be involved in the trafficking AMPAR during synaptic plasticity. Function SAP97 is expressed throughout the body in epithelial cells, including the kidney and brain. There is some evidence that SAP97 regulates cell-to-cell adhesion during cell death, and may interact with HPV. In the brain, SAP97's function is involved in the trafficking of transmembrane receptors from the ER to the plasma membrane. SAP97's function has been investigated by reducing its expr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PDZ Domain
The PDZ domain is a common structural domain of 80-90 amino-acids found in the signaling proteins of bacteria, yeast, plants, viruses and animals. Proteins containing PDZ domains play a key role in anchoring receptor proteins in the membrane to cytoskeletal components. Proteins with these domains help hold together and organize signaling complexes at cellular membranes. These domains play a key role in the formation and function of signal transduction complexes. PDZ domains also play a highly significant role in the anchoring of cell surface receptors (such as Cftr and FZD7) to the actin cytoskeleton via mediators like NHERF and ezrin. ''PDZ'' is an initialism combining the first letters of the first three proteins discovered to share the domain — post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (zo-1). PDZ domains have previously been referred to as DHR (Dlg homologous region) or GLGF (glycine-leucine-glycin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |