|
ACE2
Angiotensin-converting enzyme 2 (ACE2) is an enzyme that can be found either attached to the membrane of cells (mACE2) in the intestines, kidney, testis, gallbladder, and heart or in a soluble form (sACE2). Both membrane bound and soluble ACE2 are integral parts of the renin–angiotensin–aldosterone system (RAAS) that exists to keep the body's blood pressure in check. While mACE2 does not appear to factor into the harmful phase of RAAS (the increase of blood pressure), its existence is vital in order for the enzyme ADAM17 to cleave its extracellular domain to create soluble ACE2 (sACE2). Soluble ACE2 lowers blood pressure by catalyzing the hydrolysis of angiotensin II (a vasoconstrictor peptide) into angiotensin (1–7) (a vasodilator) which in turns binds to MasR receptors creating localized vasodilation and hence decreasing blood pressure. This decrease in blood pressure makes the entire process a promising drug target for treating cardiovascular diseases. mACE2 also serv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a provisional name, 2019 novel coronavirus (2019-nCoV), and has also been called the human coronavirus 2019 (HCoV-19 or hCoV-19). First identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a public health emergency of international concern on January 30, 2020, and a pandemic on March 11, 2020. SARS‑CoV‑2 is a positive-sense single-stranded RNA virus that is contagious in humans. SARS‑CoV‑2 is a virus of the species ''severe acute respiratory syndrome–related coronavirus'' (SARSr-CoV), related to the SARS-CoV-1 virus that caused the 2002–2004 SARS outbreak. Despite its close relation to SARS-CoV-1, its closest known relatives, with which it forms a sister group, are the derived SARS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coronaviruses
Coronaviruses are a group of related RNA viruses that cause diseases in mammals and birds. In humans and birds, they cause respiratory tract infections that can range from mild to lethal. Mild illnesses in humans include some cases of the common cold (which is also caused by other viruses, predominantly rhinoviruses), while more lethal varieties can cause SARS, MERS and COVID-19, which is causing the ongoing pandemic. In cows and pigs they cause diarrhea, while in mice they cause hepatitis and encephalomyelitis. Coronaviruses constitute the subfamily ''Orthocoronavirinae'', in the family ''Coronaviridae'', order ''Nidovirales'' and realm ''Riboviria''. They are enveloped viruses with a positive-sense single-stranded RNA genome and a nucleocapsid of helical symmetry. The genome size of coronaviruses ranges from approximately 26 to 32 kilobases, one of the largest among RNA viruses. They have characteristic club-shaped spikes that project from their surface, which in electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADAM17
A disintegrin and metalloprotease 17 (ADAM17), also called TACE (''tumor necrosis factor-α-converting enzyme''), is a 70-kDa enzyme that belongs to the ADAM protein family of disintegrins and metalloproteases. Chemical characteristics ADAM17 is an 824-amino acid polypeptide. Function ADAM17 is understood to be involved in the processing of tumor necrosis factor alpha (TNF-α) at the surface of the cell, and from within the intracellular membranes of the trans-Golgi network. This process, which is also known as 'shedding', involves the cleavage and release of a soluble ectodomain from membrane-bound pro-proteins (such as pro-TNF-α), and is of known physiological importance. ADAM17 was the first 'sheddase' to be identified, and is also understood to play a role in the release of a diverse variety of membrane-anchored cytokines, cell adhesion molecules, receptors, ligands, and enzymes. Cloning of the TNF-α gene revealed it to encode a 26 kDa type II transmembrane pro- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angiotensin (1-7)
Angiotensin (1-7) (; Molecular weight = 899.02 g/mol; H- Asp-Arg- Val- Tyr-Ile-His-Pro-OH) is an active heptapeptide of the renin–angiotensin system (RAS). In 1988, Santos ''et al'' demonstrated that angiotensin (1-7) was a main product of the incubation of angiotensin I with brain micropunches and Schiavone ''et al'' reported the first biological effect of this heptapeptide. Angiotensin (1-7) is a vasodilator agent affecting cardiovascular organs, such as heart, blood vessels and kidneys, with functions frequently opposed to those attributed to the major effector component of the RAS, angiotensin II (Ang II). Synthesis The polypeptide Ang I can be converted into Ang (1-7) by the actions of neprilysin (NEP) and thimet oligopeptidase (TOP) enzymes. Also, Ang II can be hydrolyzed into Ang (1-7) through the actions of angiotensin-converting enzyme 2 (ACE2). Ang (1-7) binds and activates the G-protein coupled receptor Mas receptor leading to opposite effects of those of Ang II. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV
Severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1; or Severe acute respiratory syndrome coronavirus, SARS-CoV) is a strain of coronavirus that causes severe acute respiratory syndrome (SARS), the respiratory illness responsible for the 2002–2004 SARS outbreak. It is an enveloped, positive-sense, single-stranded RNA virus that infects the epithelial cells within the lungs. The virus enters the host cell by binding to angiotensin-converting enzyme 2. It infects humans, bats, and palm civets. On April 16, 2003, following the outbreak of SARS in Asia and secondary cases elsewhere in the world, the World Health Organization (WHO) issued a press release stating that the coronavirus identified by a number of laboratories was the official cause of SARS. The Centers for Disease Control and Prevention (CDC) in the United States and the National Microbiology Laboratory (NML) in Canada identified the SARS-CoV-1 genome in April 2003. Scientists at Erasmus University in R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
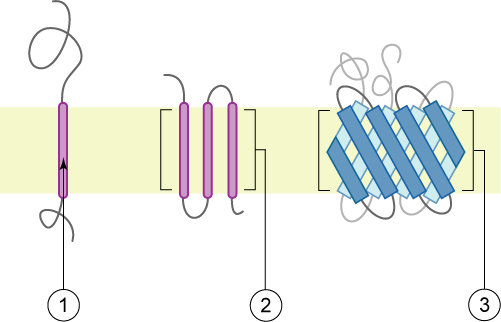
Transmembrane Domain
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs generally adopt an alpha helix topological conformation, although some TMDs such as those in porins can adopt a different conformation. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in length, sequence, and hydrophobicity, adopting organelle-specific properties. Functions of transmembrane domains Transmembrane domains are known to perform a variety of functions. These include: * Anchoring transmembrane proteins to the membrane. *Facilitating molecular transport of molecules such as ions and proteins across biological membranes; usually hydrophilic residues and binding sites in the TMDs help in this process. *Signal transduction across the membrane; many transmembrane proteins, such as G protein-coupled receptors, receive extracellular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type I Membrane Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to the network of interactions between different proteins in cells, including interactions via transmembrane alpha helices. They usually include one or several water-soluble domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. A 2013 estimate identified about 1300 single-pass membrane proteins in the human genome. Topology-based classification Bitopic proteins are classified into 4 types, depending on their transmembrane topology and location of the transmembrane heli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid Transporter
An amino acid transporter is a membrane transport protein that transports amino acids. They are mainly of the solute carrier family. Families There are several families that function in amino acid transport, some of these include: TC# 2.A.3- Amino Acid-Polyamine-Organocation (APC) Superfamily TC# 2.A.18- Amino Acid/Auxin Permease (AAAP) Family TC# 2.A.23- Dicarboxylate/Amino Acid:Cation (Na+ or H+) Symporter (DAACS) Family TC# 2.A.26- Branched Chain Amino Acid:Cation Symporter (LIVCS) Family TC# 2.A.42- Hydroxy/Aromatic Amino Acid Permease (HAAAP) Family TC# 2.A.78- Branched Chain Amino Acid Exporter (LIV-E) Family TC# 2.A.95- 6TMS Neutral Amino Acid Transporter (NAAT) Family TC# 2.A.118- Basic Amino Acid Antiporter (ArcD) Family TC# 2.A.120- Putative Amino Acid Permease (PAAP) Family Solute carrier family examples * (1) high affinity glutamate and neutral amino acid transporter * (3) heavy subunits of heteromeric amino acid transporters * (6) Bacterial Leucine Transp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_synthesis_pathway.png)

.png)