 |
A15 Phases
The A15 phases (also known as β-W or Cr3Si structure types) are series of intermetallic compounds with the chemical formula ''A''3''B'' (where A is a transition metal and B can be any element) and a specific structure. The A15 phase is also one of the members in the Frank–Kasper phases family. Many of these compounds have superconductivity at around , which is comparatively high, and remain superconductive in magnetic fields of tens of teslas (hundreds of kilogauss). This kind of superconductivity ( Type-II superconductivity) is an important area of study as it has several practical applications. History The first time that A15 structure was observed was in 1931 when an electrolytically deposited layer of tungsten was examined. Discussion of whether the β-tungsten structure is an allotrope of tungsten or the structure of a tungsten suboxide was long-standing, but since the 1950s there has been many publications showing that the material is a true allotrope of tungsten. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Cuprate Superconductor
Cuprate superconductors are a family of high-temperature superconducting materials made of layers of copper oxides (CuO2) alternating with layers of other metal oxides, which act as charge reservoirs. At ambient pressure, cuprate superconductors are the highest temperature superconductors known. However, the mechanism by which superconductivity occurs is still not understood. History The first cuprate superconductor was found in 1986 in the non-stoichiometric cuprate lanthanum barium copper oxide by IBM researchers Georg Bednorz and Karl Alex Müller. The critical temperature for this material was 35K, well above the previous record of 23 K. The discovery led to a sharp increase in research on the cuprates, resulting in thousands of publications between 1986 and 2001. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987, only a year after their discovery. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Intermetallics
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic intermetallic compounds. Although the term "intermetallic compounds", as it applies to solid phases, has been in use for many years, its introduction was regretted, for example by Hume-Rothery in 1955. Definitions Research definition Schulze in 1967 defined intermetallic compounds as ''solid phases containing two or more metallic elements, with optionally one or more non-metallic elements, whose crystal structure differs from that of the other constituents''. Under this definition, the following are included: #Electron (or Hume-Rothery) compounds #Size packing phases. e.g. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements barring carbon (which sublimes at normal pressure), melting at . It also has the highest boiling point, at . Its density is , comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Titanium Gold
In metallurgy, titanium gold (Ti-Au or Au-Ti) refers to an alloy consisting of titanium and gold. Such alloys are used in dentistry, ceramics and jewelry. Like many other alloys, titanium gold alloys have a higher yield strength, tensile strength, hardness, and magnetism than either of its constituent metals. In July 2016, researchers discovered that a titanium-gold alloy, β-Ti3Au (strictly speaking, an intermetallic), is up to 4 times harder than titanium. In popular culture In the 2008 film ''Iron Man'', the title character wears an armor made from a titanium-gold alloy. According to actor Robert Downey Jr., who played the role of Iron Man, the suit fit him "like a gold-titanium glove".http://www.theinsider.com/news/842818_Robert_Downey_Jr._Says_Iron_Man_Suit_Fits_Him_Like_Gold_Titanium_Gold In the 2019 book '' The Secret Commonwealth'' (part of the universe of His Dark Materials trilogy), the alethiometer is revealed to be made primarily of a titanium-gold alloy. In S6:E22 o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Clathrate Hydrates
Clathrate hydrates, or gas hydrates, clathrates, hydrates, etc., are crystalline water-based solids physically resembling ice, in which small Chemical polarity, non-polar molecules (typically gases) or Chemical polarity, polar molecules with large hydrophobic Moiety (chemistry), moieties are trapped inside "cages" of hydrogen bonded, frozen water (molecule), water molecules. In other words, clathrate hydrates are clathrate compounds in which the host molecule is water and the guest molecule is typically a gas or liquid. Without the support of the trapped molecules, the Crystal structure , lattice structure of hydrate clathrates would collapse into conventional ice crystal structure or liquid water. Most low molecular weight gases, including , , , carbon dioxide, , methane, , hydrogen sulfide, , , , and , as well as some higher hydrocarbons and freons, will form hydrates at suitable temperatures and pressures. Clathrate hydrates are not officially chemical compounds, as the enclath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
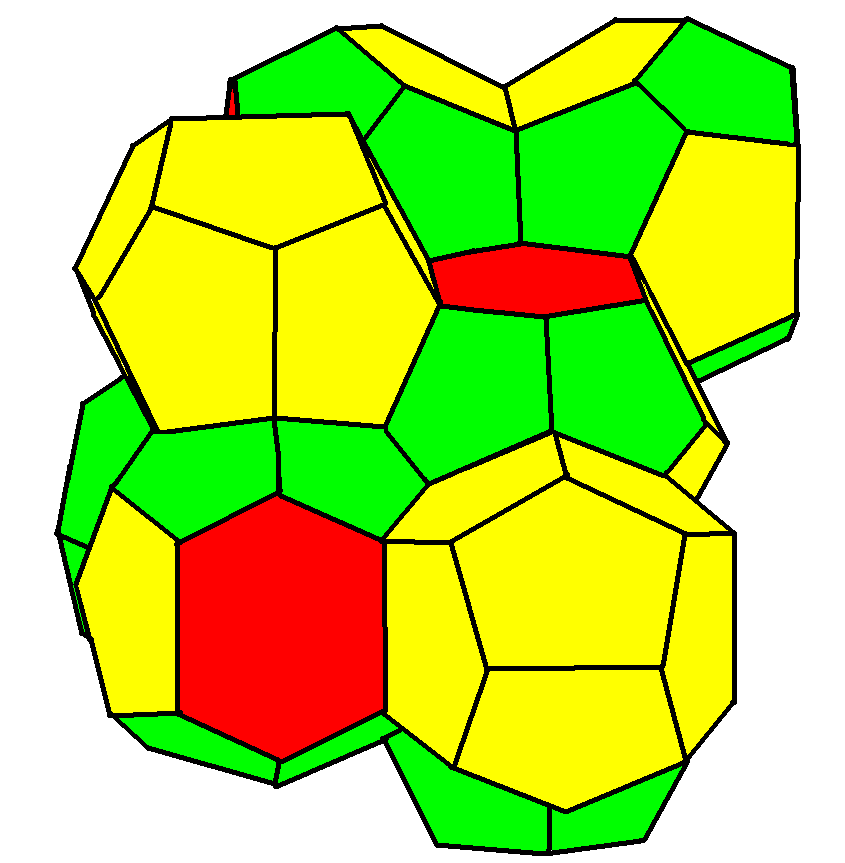 |
Weaire–Phelan Structure
In geometry, the Weaire–Phelan structure is a three-dimensional structure representing an idealised foam of equal-sized bubbles, with two different shapes. In 1993, Denis Weaire and Robert Phelan found that this structure was a better solution of the Kelvin problem of tiling space by equal volume cells of minimum surface area than the previous best-known solution, the Kelvin structure. History and the Kelvin problem In two dimensions, the subdivision of the plane into cells of equal area with minimum average perimeter is given by the hexagonal tiling, but although the first record of this honeycomb conjecture goes back to the ancient Roman scholar Marcus Terentius Varro, it was not proven until the work of Thomas C. Hales in 1999. In 1887, Lord Kelvin asked the corresponding question for three-dimensional space: how can space be partitioned into cells of equal volume with the least area of surface between them? Or, in short, what was the most efficient soap bubble foam? Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Voronoi Diagram
In mathematics, a Voronoi diagram is a partition of a plane into regions close to each of a given set of objects. In the simplest case, these objects are just finitely many points in the plane (called seeds, sites, or generators). For each seed there is a corresponding region, called a Voronoi cell, consisting of all points of the plane closer to that seed than to any other. The Voronoi diagram of a set of points is dual to that set's Delaunay triangulation. The Voronoi diagram is named after mathematician Georgy Voronoy, and is also called a Voronoi tessellation, a Voronoi decomposition, a Voronoi partition, or a Dirichlet tessellation (after Peter Gustav Lejeune Dirichlet). Voronoi cells are also known as Thiessen polygons. Voronoi diagrams have practical and theoretical applications in many fields, mainly in science and technology, but also in visual art. The simplest case In the simplest case, shown in the first picture, we are given a finite set of points in the Euclidean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |