|
Azurin (Dungeons
Azurin is a small, periplasmic, bacterial blue copper protein found in '' Pseudomonas'', '' Bordetella'', or '' Alcaligenes'' bacteria. Azurin moderates single-electron transfer between enzymes associated with the cytochrome chain by undergoing oxidation-reduction between Cu(I) and Cu(II). Each monomer of an azurin tetramer has a molecular weight of approximately 14kDa, contains a single copper atom, is intensively blue, and has a fluorescence emission band centered at 308 nm. Azurins and pseudoazurins participate in the denitrification processes in bacteria., including the gram-negative bacteria '' Pseudomonas aeruginosa,'' by interacting with cytochrome c551. Azurin from ''P aeruginosa'' is a type I blue copper protein (cupredoxin), while cytochrome c551 (9 kDa) is a haem-containing cytochrome. Azurin possesses a relatively large hydrophobic patch close to the active site, and two residues in this hydrophobic patch, Met-44 and Met-64, are believed to be involved in its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Protein
Copper proteins are proteins that contain one or more copper ions as prosthetic groups. Copper proteins are found in all forms of air-breathing life. These proteins are usually associated with electron-transfer with or without the involvement of oxygen (O2). Some organisms even use copper proteins to carry oxygen instead of iron proteins. A prominent copper proteins in humans is in cytochrome c oxidase (cco). The enzyme cco mediates the controlled combustion that produces ATP. Classes The metal centers in the copper proteins can be classified into several types: *Type I copper centres (T1Cu) are characterized by a single copper atom coordinated by two histidine residues and a cysteine residue in a trigonal planar structure, and a variable axial ligand. In class I T1Cu proteins (e.g. amicyanin, plastocyanin and pseudoazurin) the axial ligand is the sulfur of methionine, whereas aminoacids other than methionine (e.g. glutamine) give rise to class II T1Cu copper proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pi Backbonding
In chemistry, π backbonding, also called π backdonation, is when electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a ''π-acceptor ligand''. It is especially common in the organometallic chemistry of transition metals with multi-atomic ligands such as carbon monoxide, ethylene or the nitrosonium cation. Electrons from the metal are used to bond to the ligand, in the process relieving the metal of excess negative charge. Compounds where π backbonding occurs include Ni(CO)4 and Zeise's salt. IUPAC offers the following definition for backbonding: A description of the bonding of π-conjugated ligands to a transition metal which involves a synergic process with donation of electrons from the filled π-orbital or lone electron pair orbital of the ligand into an empty orbital of the metal (donor–acceptor bond), together with release (back donation) of electrons from an ''n''d orbital of the metal (which is of π-symmetry with res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angiogenesis
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature by processes of sprouting and splitting. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise (especially in older texts). The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease. Angiogenesis is a normal and vital process in growth and development, as well as in wound healing and in the formation of granulation tissue. However, it is also a fundamental step in the transition of tumors from a benign state to a malignant one, leading to the use of angiogenesis inhibitors in the treatment of cancer. The essential role of angiogenesis in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
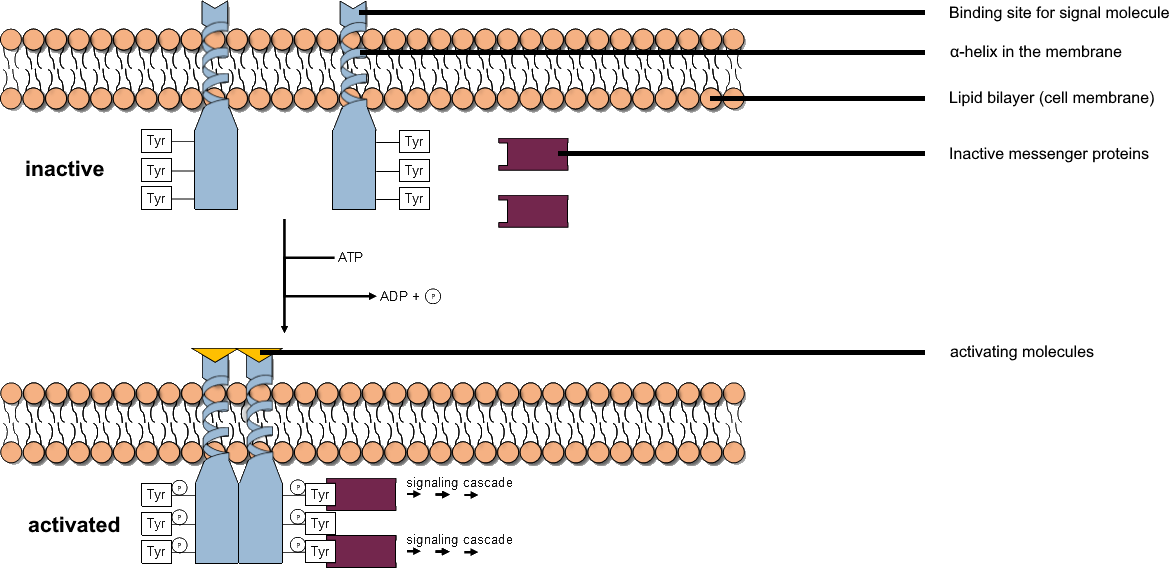
Tyrosine Kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. Phosphorylation of proteins by kinases is an important mechanism for communicating signals within a cell (signal transduction) and regulating cellular activity, such as cell division. Protein kinases can become mutated, stuck in the "on" position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases have an associated protein tyrosine phosphatase, which removes the phosphate group. Reaction Protein kinases are a group of enzymes that possess a catal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glioma
A glioma is a type of tumor that starts in the glial cells of the brain or the spine. Gliomas comprise about 30 percent of all brain tumors and central nervous system tumours, and 80 percent of all malignant brain tumours. Signs and symptoms Symptoms of gliomas depend on which part of the central nervous system is affected. A brain glioma can cause headaches, vomiting, seizures, and cranial nerve disorders as a result of increased intracranial pressure. A glioma of the optic nerve can cause visual loss. Spinal cord gliomas can cause pain, weakness, or numbness in the extremities. Gliomas do not usually metastasize by the bloodstream, but they can spread via the cerebrospinal fluid and cause "drop metastases" to the spinal cord. Complex visual hallucinations have been described as a symptom of low-grade glioma. A child who has a subacute disorder of the central nervous system that produces cranial nerve abnormalities (especially of cranial nerve VII and the lower bulbar nerv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G1/S Transition
The G1/S transition is a stage in the cell cycle at the boundary between the G1 phase, in which the cell grows, and the S phase, during which DNA is replicated. It is governed by cell cycle checkpoints to ensure cell cycle integrity and the subsequent S phase can pause in response to improperly or partially replicated DNA. During this transition the cell makes decisions to become quiescent (enter G0), differentiate, make DNA repairs, or proliferate based on environmental cues and molecular signaling inputs. The G1/S transition occurs late in G1 and the absence or improper application of this highly regulated check point can lead to cellular transformation and disease states such as cancer During this transition, G1 cyclin D-Cdk4/6 dimer phosphorylates retinoblastoma releasing transcription factor E2F, which then drives the transition from G1 to S phase. The G1/S transition is highly regulated by transcription factor p53 in order to halt the cell cycle when DNA is damaged. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noxa
Phorbol-12-myristate-13-acetate-induced protein 1 is a protein that in humans is encoded by the ''PMAIP1'' gene, and is also known as Noxa. Noxa (Latin for ''damage'') is a pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members can form hetero- or homodimers, and they act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. The expression of Noxa is regulated by the tumor suppressor p53, and Noxa has been shown to be involved in p53-mediated apoptosis. Interactions Noxa has been shown to interact with: * BCL2-like 1, * Bcl-2, and * MCL1. See also * Apoptosis * Apoptosome * Bcl-2 * Bcl-2-associated X protein (BAX) * BH3 interacting domain death agonist (BID) * Caspases * Cytochrome c * Mitochondrion * p53 upregulated modulator of apoptosis The p53 upregulated modulator of apoptosis (PUMA) also known as Bcl-2-binding component 3 (BBC3), is a pro-apoptotic protein, member of the Bcl-2 protein family. In humans, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BAX (gene)
Apoptosis regulator BAX, also known as bcl-2-like protein 4, is a protein that in humans is encoded by the ''BAX'' gene. ''BAX'' is a member of the Bcl-2 gene family. BCL2 family members form hetero- or homodimers and act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. This protein forms a heterodimer with BCL2, and functions as an apoptotic activator. This protein is reported to interact with, and increase the opening of, the mitochondrial voltage-dependent anion channel (VDAC), which leads to the loss in membrane potential and the release of cytochrome c. The expression of this gene is regulated by the tumor suppressor P53 and has been shown to be involved in P53-mediated apoptosis. Structure The ''BAX'' gene was the first identified pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains (named BH1, BH2, B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
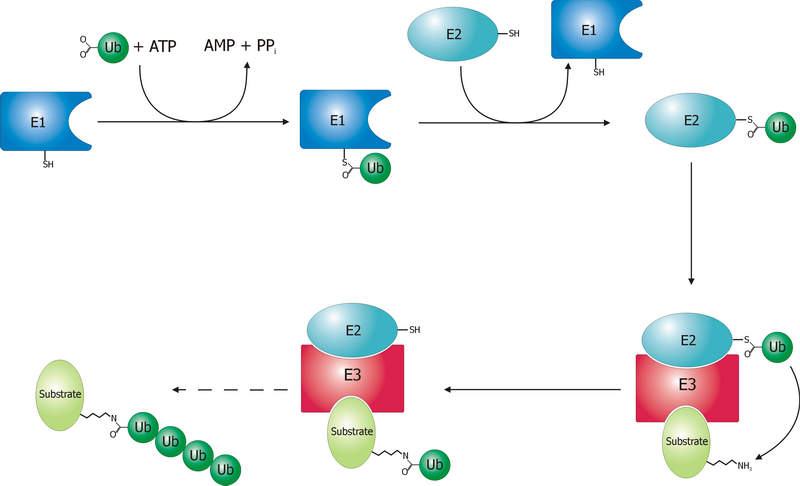
Ubiquitin Ligase
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquitin from the E2 to the protein substrate. In simple and more general terms, the ligase enables movement of ubiquitin from a ubiquitin carrier to another thing (the substrate) by some mechanism. The ubiquitin, once it reaches its destination, ends up being attached by an isopeptide bond to a lysine residue, which is part of the target protein. E3 ligases interact with both the target protein and the E2 enzyme, and so impart substrate specificity to the E2. Commonly, E3s polyubiquitinate their substrate with Lys48-linked chains of ubiquitin, targeting the substrate for destruction by the proteasome. However, many other types of linkages are possible and alter a protein's activity, interactions, or localization. Ubiquitination by E3 ligases reg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C Oxidase
The enzyme cytochrome c oxidase or Complex IV, (was , now reclassified as a translocasEC 7.1.1.9 is a large transmembrane protein complex found in bacteria, archaea, and mitochondria of eukaryotes. It is the last enzyme in the respiratory electron transport chain of cells located in the membrane. It receives an electron from each of four cytochrome c molecules and transfers them to one oxygen molecule and four protons, producing two molecules of water. In addition to binding the four protons from the inner aqueous phase, it transports another four protons across the membrane, increasing the transmembrane difference of proton electrochemical potential, which the ATP synthase then uses to synthesize ATP. Structure The complex The complex is a large integral membrane protein composed of several metal prosthetic sites and 14 protein subunits in mammals. In mammals, eleven subunits are nuclear in origin, and three are synthesized in the mitochondria. The complex contains two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine Dehydrogenase
Amine Dehydrogenase (), also known as methylamine dehydrogenase (MADH), is a tryptophan tryptophylquinone-dependent (TTQ-dependent) enzyme that catalyzes the oxidative deamination of a primary amine to an aldehyde and ammonia. The reaction occurs as follows: RCH2NH2 + H2O + acceptor → RCHO + NH3 + reduced acceptor Amine dehydrogenase possesses an α2β2 structure with each smaller β subunit possessing a TTQ protein cofactor. Amine dehydrogenase, studied in ''Paracoccus denitrificans'', at least transiently forms a ternary complex to catalyze methylamine-dependent cytochrome c-551i reduction. Within this complex, electrons are transferred from the TTQ cofactor of MADH to the Type 1 copper center of amicyanin, and then to the heme of the cytochrome Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of hem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Deamination
Oxidative deamination is a form of deamination that generates α-keto acids and other oxidized products from amine-containing compounds, and occurs primarily in the liver. Oxidative deamination is stereospecific, meaning it contains different stereoisomers as reactants and products; this process is either catalyzed by L or D- amino acid oxidase and L-amino acid oxidase is present only in the liver and kidney. Oxidative deamination is an important step in the catabolism of amino acids, generating a more metabolizable form of the amino acid, and also generating ammonia as a toxic byproduct. The ammonia generated in this process can then be neutralized into urea via the urea cycle. Much of the oxidative deamination occurring in cells involves the amino acid glutamate, which can be oxidatively deaminated by the enzyme glutamate dehydrogenase (GDH), using NAD or NADP as a coenzyme. This reaction generates α-ketoglutarate (α-KG) and ammonia. Glutamate can then be regenerated from α-K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |