|
8-aminoquinoline
8-Aminoquinoline is the 8-amino derivative of quinoline. Often abbreviated AQ, it is a pale yellow solid. It is structurally analogous to 8-hydroxyquinoline. Drug derivatives The derivatives primaquine, tafenoquine and pamaquine have been tested for anti-malaria activity. Primaquine is still used routinely worldwide as part of the treatment of ''Plasmodium vivax'' and ''Plasmodium ovale'' malaria, although how it prevents malarial recurrences is not, at present, clear. Tafenoquine was approved for medical use in Australia and in the United States in 2018. Image:Primaquine.svg, Primaquine Image:Pamaquine.svg, Pamaquine Image:Tafenoquine.svg, Tafenoquine Directing group The amine functional group is amenable to formation of amide In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pamaquine
Pamaquine is an 8-aminoquinoline drug formerly used for the treatment of malaria. It is closely related to primaquine. Synonyms *Plasmochin *Plasmoquine *Plasmaquine Uses Pamaquine is effective against the hypnozoites of the relapsing malarias ('' P. vivax'' and '' P. ovale''); and unlike primaquine, it is also very effective against the erythrocytic stages of all four human malarias. One small clinical trial of pamaquine as a causal prophylactic was disappointing (whereas primaquine is an extremely effective causal prophylactic). Pamaquine is more toxic and less efficacious than primaquine; therefore, pamaquine is no longer routinely used, and of the two, only primaquine is currently recommended by the World Health Organization. Adverse effects Like primaquine, pamaquine causes haemolytic anaemia in patients with G6PD deficiency. Patients should therefore always be screened for G6PD deficiency prior to being prescribed pamaquine. History Pamaquine was the second synthetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
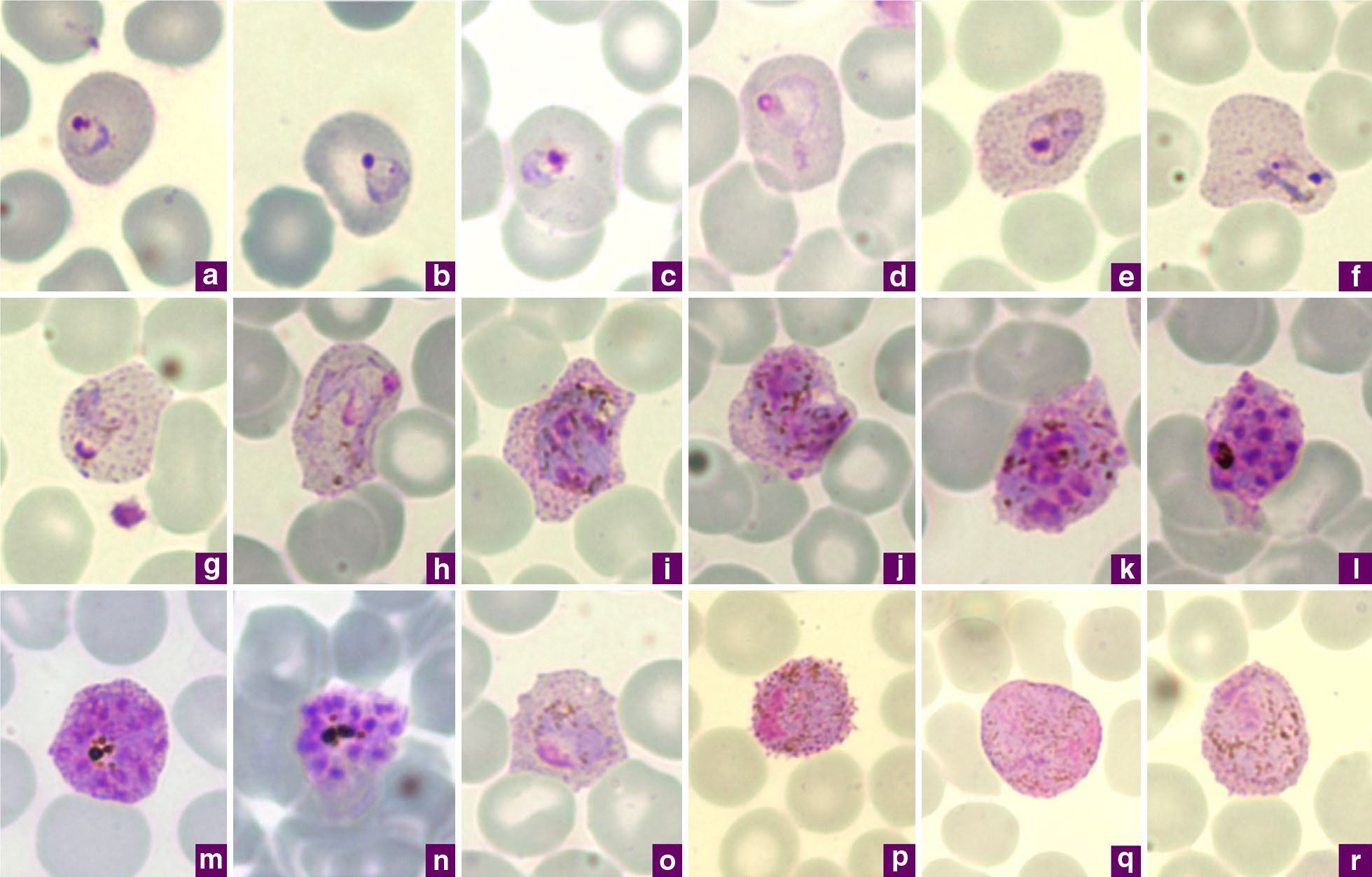
Malaria
Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin ten to fifteen days after being bitten by an infected mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria. Malaria is caused by single-celled microorganisms of the ''Plasmodium'' group. It is spread exclusively through bites of infected ''Anopheles'' mosquitoes. The mosquito bite introduces the parasites from the mosquito's saliva into a person's blood. The parasites travel to the liver where they mature and reproduce. Five species of ''Plasmodium'' can infect and be spread by h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pamaquine
Pamaquine is an 8-aminoquinoline drug formerly used for the treatment of malaria. It is closely related to primaquine. Synonyms *Plasmochin *Plasmoquine *Plasmaquine Uses Pamaquine is effective against the hypnozoites of the relapsing malarias ('' P. vivax'' and '' P. ovale''); and unlike primaquine, it is also very effective against the erythrocytic stages of all four human malarias. One small clinical trial of pamaquine as a causal prophylactic was disappointing (whereas primaquine is an extremely effective causal prophylactic). Pamaquine is more toxic and less efficacious than primaquine; therefore, pamaquine is no longer routinely used, and of the two, only primaquine is currently recommended by the World Health Organization. Adverse effects Like primaquine, pamaquine causes haemolytic anaemia in patients with G6PD deficiency. Patients should therefore always be screened for G6PD deficiency prior to being prescribed pamaquine. History Pamaquine was the second synthetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primaquine
Primaquine is a medication used to treat and prevent malaria and to treat ''Pneumocystis'' pneumonia. Specifically it is used for malaria due to ''Plasmodium vivax'' and ''Plasmodium ovale'' along with other medications and for prevention if other options cannot be used. It is an alternative treatment for ''Pneumocystis'' pneumonia together with clindamycin. It is taken by mouth. Common side effects include nausea, vomiting, and stomach cramps. Primaquine should not be given to people with glucose-6-phosphate dehydrogenase (G6PD) deficiency due to the risk of red blood cell breakdown. It is often recommended that primaquine not be used during pregnancy. It may be used while breastfeeding if the baby is known not to have G6PD deficiency. The mechanisms of action is not entirely clear but is believed to involve effects on the malaria parasites' DNA. Primaquine was first made in 1946. It is on the World Health Organization's List of Essential Medicines. It is available as a gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primaquine
Primaquine is a medication used to treat and prevent malaria and to treat ''Pneumocystis'' pneumonia. Specifically it is used for malaria due to ''Plasmodium vivax'' and ''Plasmodium ovale'' along with other medications and for prevention if other options cannot be used. It is an alternative treatment for ''Pneumocystis'' pneumonia together with clindamycin. It is taken by mouth. Common side effects include nausea, vomiting, and stomach cramps. Primaquine should not be given to people with glucose-6-phosphate dehydrogenase (G6PD) deficiency due to the risk of red blood cell breakdown. It is often recommended that primaquine not be used during pregnancy. It may be used while breastfeeding if the baby is known not to have G6PD deficiency. The mechanisms of action is not entirely clear but is believed to involve effects on the malaria parasites' DNA. Primaquine was first made in 1946. It is on the World Health Organization's List of Essential Medicines. It is available as a gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tafenoquine
Tafenoquine, sold under the brand name Krintafel among others, is a medication used to prevent and to treat malaria. With respect to acute malaria, it is used together with other medications to prevent relapse by ''Plasmodium vivax''. It may be used to prevent all types of malaria. It is taken by mouth. Common side effects include vomiting, headache, and dizziness. Other side effects may include methemoglobinemia, trouble sleeping, and anaphylaxis. In people with G6PD deficiency, red blood cell breakdown may occur. Use in pregnancy is not recommended. Tafenoquine is in the 8-aminoquinoline family of medications. How it works is unclear but it is effective both in the liver and bloodstream. A possible mechanism of action and other novel perspectives have been published. Tafenoquine was approved for medical use in Australia and in the United States in 2018. Tafenoquine is related to primaquine. Medical use Prevention Tafenoquine may be used to prevent all types of malaria. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasmodium Vivax
''Plasmodium vivax'' is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than ''Plasmodium falciparum'', the deadliest of the five human malaria parasites, ''P. vivax'' malaria infections can lead to severe disease and death, often due to splenomegaly (a pathologically enlarged spleen). ''P. vivax'' is carried by the female ''Anopheles'' mosquito; the males do not bite. Health Epidemiology ''Plasmodium vivax'' is found mainly in Asia, Latin America, and in some parts of Africa. ''P. vivax'' is believed to have originated in Asia, but recent studies have shown that wild chimpanzees and gorillas throughout central Africa are endemically infected with parasites that are closely related to human ''P. vivax.'' These findings indicate that human P. vivax is of African origin. ''Plasmodium vivax'' accounts for 65% of malaria cases in Asia and South America. Unlike ''Pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tafenoquine
Tafenoquine, sold under the brand name Krintafel among others, is a medication used to prevent and to treat malaria. With respect to acute malaria, it is used together with other medications to prevent relapse by ''Plasmodium vivax''. It may be used to prevent all types of malaria. It is taken by mouth. Common side effects include vomiting, headache, and dizziness. Other side effects may include methemoglobinemia, trouble sleeping, and anaphylaxis. In people with G6PD deficiency, red blood cell breakdown may occur. Use in pregnancy is not recommended. Tafenoquine is in the 8-aminoquinoline family of medications. How it works is unclear but it is effective both in the liver and bloodstream. A possible mechanism of action and other novel perspectives have been published. Tafenoquine was approved for medical use in Australia and in the United States in 2018. Tafenoquine is related to primaquine. Medical use Prevention Tafenoquine may be used to prevent all types of malaria. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Directing Group
In organic chemistry, a directing group (DG) is a substituent on a molecule or ion that facilitates reactions by interacting with a reagent. The term is usually applied to C-H activation of hydrocarbons, where it is defined as a "coordinating moiety (an “internal ligand”), which directs a metal catalyst into the proximity of a certain C–H bond." In a well known example, the ketone group () in acetophenone is the DG in the Murai reaction. The Murai reaction is related to directed ortho metalation, a reaction is typically applied to the lithiation of substituted aromatic rings.''Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics Victor Snieckus'' Chem. Rev.; 1990; 90(6); 879-933Abstract/ref> A wide variety of functional groups can serve as directing groups. Transient directing groups Since directing groups are ligands, their effectiveness correlates with their affinities for metals. Common fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abandoned Drugs
Abandon, abandoned, or abandonment may refer to: Common uses * Abandonment (emotional), a subjective emotional state in which people feel undesired, left behind, insecure, or discarded * Abandonment (legal), a legal term regarding property ** Child abandonment, the extralegal abandonment of children ** Lost, mislaid, and abandoned property, legal status of property after abandonment and rediscovery * Abandonment (mysticism) Art, entertainment, and media Film * ''Abandon'' (film), a 2002 film starring Katie Holmes * ''Abandoned'' (1949 film), starring Dennis O'Keefe * ''Abandoned'' (1955 film), the English language title of the Italian war film ''Gli Sbandati'' * ''Abandoned'' (2001 film), a Hungarian film * ''Abandoned'' (2010 film), starring Brittany Murphy * ''Abandoned'' (2015 film), a television movie about the shipwreck of the ''Rose-Noëlle'' in 1989 * ''Abandoned'' (2022 film), starring Emma Roberts * ''The Abandoned'' (1945 film), a 1945 Mexican film * ''The Aban ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance. Occurrence and isolation Quinoline was first extracted from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge; he called quinoline ''leukol'' ("white oil" in Greek). Coal tar remains the principal source of commercial quinoline. In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, stry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |