|
6-nitroquipazine
6-Nitroquipazine (developmental code name DU-24,565) is a potent and selective serotonin reuptake inhibitor used in scientific research. 6-Nitroquipazine has been found to inhibit melanogenesis ''in-vitro'', suggesting that it can be used for skin whitening. See also * Quipazine Quipazine is a serotonergic drug of the piperazine group which is used in scientific research. It was originally intended as an antidepressant but never developed for medical use. Pharmacology Pharmacodynamics Quipazine is a serotonin reuptake ... References {{DEFAULTSORT:Nitroquipazine, 6- Nitro compounds Piperazines Quinolines Serotonin reuptake inhibitors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potent (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
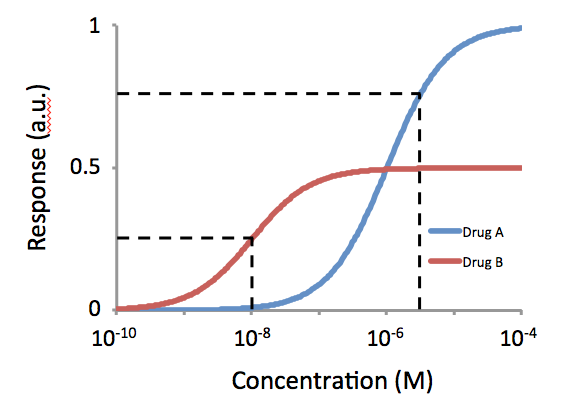
Functional Selectivity
Functional selectivity (or “agonist trafficking”, “biased agonism”, “biased signaling”, "ligand bias" and “differential engagement”) is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand (often the endogenous hormone or peptide) at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the μ-opioid re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Reuptake Inhibitor
A serotonin reuptake inhibitor (SRI) is a type of drug which acts as a reuptake inhibitor of the neurotransmitter serotonin (5-hydroxytryptamine, or 5-HT) by blocking the action of the serotonin transporter (SERT). This in turn leads to increased extracellular concentrations of serotonin and, therefore, an increase in serotonergic neurotransmission. It is a type of monoamine reuptake inhibitor (MRI); other types of MRIs include dopamine reuptake inhibitors and norepinephrine reuptake inhibitors. SRIs are not synonymous with selective serotonin reuptake inhibitors (SSRIs), as the latter term is usually used to describe the class of antidepressants of the same name, and because SRIs, unlike SSRIs, can either be selective or non-selective in their action. For example, cocaine, which non-selectively inhibits the reuptake of serotonin, norepinephrine, and dopamine, is an SRI but not an SSRI. SRIs are used predominantly as antidepressants (e.g., SSRIs, SNRIs, and TCAs), though they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scientific Research
The scientific method is an empirical method for acquiring knowledge that has characterized the development of science since at least the 17th century (with notable practitioners in previous centuries; see the article history of scientific method for additional detail.) It involves careful observation, applying rigorous skepticism about what is observed, given that cognitive assumptions can distort how one interprets the observation. It involves formulating hypotheses, via induction, based on such observations; the testability of hypotheses, experimental and the measurement-based statistical testing of deductions drawn from the hypotheses; and refinement (or elimination) of the hypotheses based on the experimental findings. These are ''principles'' of the scientific method, as distinguished from a definitive series of steps applicable to all scientific enterprises. Although procedures vary from one field of inquiry to another, the underlying process is frequently the sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanogenesis
Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer (the stratum basale) of the skin's epidermis, the middle layer of the eye (the uvea), the inner ear, vaginal epithelium, meninges, bones, and heart. Melanin is a dark pigment primarily responsible for skin color. Once synthesized, melanin is contained in special organelles called melanosomes which can be transported to nearby keratinocytes to induce pigmentation. Thus darker skin tones have more melanosomes present than lighter skin tones. Functionally, melanin serves as protection against UV radiation. Melanocytes also have a role in the immune system. Function Through a process called melanogenesis, melanocytes produce melanin, which is a pigment found in the skin, eyes, hair, nasal cavity, and inner ear. This melanogenesis leads to a long-lasting pigmentation, which is in contrast to the pigmentation that originates from oxidation of already-existing melanin. There are both ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In-vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from ''in vitro'' experiments may not fully or accurately predict the effects on a whole organism. In contrast to ''in vitro'' experiments, ''in vivo'' studies are those conducted in living organisms, including humans, and whole plants. Definition ''In vitro'' ( la, in glass; often not italicized in English usage) studies are conducted using components of an organism that have been isolated fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skin Whitening
Skin whitening, also known as skin lightening and skin bleaching, is the practice of using chemical substances in an attempt to lighten the skin or provide an even skin color by reducing the melanin concentration in the skin. Several chemicals have been shown to be effective in skin whitening, while some have proven to be toxic or have questionable safety profiles. This includes mercury compounds which may cause neurological problems and kidney problems. In a number of African countries, between 25 and 80% of women regularly use skin whitening products. In Asia, this number is around 40%. In India, specifically, over half of skin care products are sold to whiten skin. In Pakistan, where skin lightening products are popular, creams have been found to contain toxic levels of hydroquinone and mercury. Efforts to lighten the skin date back to at least the 1500s in Asia. While a number of agents—such as kojic acid and alpha hydroxy acid—are allowed in cosmetics in Europe, a numb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quipazine
Quipazine is a serotonergic drug of the piperazine group which is used in scientific research. It was originally intended as an antidepressant but never developed for medical use. Pharmacology Pharmacodynamics Quipazine is a serotonin reuptake inhibitor, and also a moderately selective serotonin receptor agonist, binding to a range of different serotonin receptors, but particularly to the 5-HT2A and 5-HT3 subtypes. Quipazine produces a head-twitch response and other psychedelic-consistent effects in animal studies including in mice, rats, and monkeys. However, it failed to produce psychedelic effects in humans at a dose of 25 mg, which was the highest dose tested due to 5-HT3 mediated side effects of nausea and gastrointestinal discomfort. However Alexander Shulgin claimed that a fully effective psychedelic dose could be reached by blocking 5-HT3 receptors using a 5-HT3 antagonist. Chemistry Quipazine is synthesized by reacting 2-chloroquinoline with piperazine. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitro Compounds
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid. Synthesis Preparation of aromatic nitro compounds Aromatic nitro compounds are typically synthesized by nitration. Nitration is achieved using a mixture of nitric acid and sulfuric acid, which produce the nitronium ion (), which is the electrophile: + The nitration product produced on the la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperazines
Substituted piperazines are a class of chemical compounds based on a piperazine core. Some are used as recreational drugs and some are used in scientific research. List of substituted piperazines Benzylpiperazines File:Benzylpiperazine.svg, 1-Benzylpiperazine File:MBZP.svg, 1-Methyl-4-benzylpiperazine File:DBZP.svg, 1,4-Dibenzylpiperazine File:MDBZP.svg, 3,4-Methylenedioxy-1-benzylpiperazine File:2C-B-BZP.svg, 4-Bromo-2,5-dimethoxy-1-benzylpiperazine File:Methoxypiperamide.png, Methoxypiperamide File:Sunifiram.svg , Sunifiram File:3-Methylbenzylpiperazine structure.png, 3-Methylbenzylpiperazine * 1-Benzylpiperazine (BZP) * 1-Methyl-4-benzylpiperazine (MBZP) * 1,4-Dibenzylpiperazine (DBZP) * 3,4-Methylenedioxy-1-benzylpiperazine (MDBZP) * 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) * Methoxypiperamide (MeOP, MEXP) ((4-methoxyphenyl)(4-methylpiperazin-1-yl)methanone) * Sunifiram (1-benzoyl-4-propanoylpiperazine) * 3-Methylbenzylpiperazine (3-MeBZP) Befuraline, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolines
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance. Occurrence and isolation Quinoline was first extracted from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge; he called quinoline ''leukol'' ("white oil" in Greek). Coal tar remains the principal source of commercial quinoline. In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |