|
5α-Dihydronandrolone
5α-Dihydronandrolone (also known as 5α-DHN, dihydronandrolone, DHN, 5α-dihydro-19-nortestosterone, or 5α-estran-17β-ol-3-one) is a naturally occurring anabolic–androgenic steroid (AAS) and a 5α-reduced derivative of nandrolone (19-nortestosterone). It is a major metabolite of nandrolone and is formed from it by the actions of the enzyme 5α-reductase analogously to the formation of dihydrotestosterone (DHT) from testosterone. When testosterone is 5α-reduced into DHT, which is a much more potent AAS in comparison, its effects are potentiated on a local level. The tissues in which this occurs (i.e., the tissues that express 5α-reductase) are referred to as "androgenic" tissues and include the skin, hair follicles, and prostate gland, among others. The conversion of testosterone into DHT is an important factor in the etiology of a variety of androgen-dependent conditions, including acne, excessive facial/body hair growth, scalp hair loss, prostate enlargement, and prostat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nandrolone
Nandrolone, also known as 19-nortestosterone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as nandrolone decanoate (brand name Deca-Durabolin) and nandrolone phenylpropionate (brand name Durabolin). Nandrolone esters are used in the treatment of anemias, cachexia (muscle wasting syndrome), osteoporosis, breast cancer, and for other indications. They are not used by mouth and instead are given by injection into muscle or fat. Side effects of nandrolone esters include symptoms of masculinization like acne, increased hair growth, voice changes, and decreased sexual desire due to its ability to suppress endogenous testosterone synthesis while not being a sufficient androgen itself. They are synthetic androgens and anabolic steroids and hence are agonists of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). Nandrolone has strong anabolic effects and weak androgenic effects, which giv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5α-Dihydrolevonorgestrel
5α-Dihydrolevonorgestrel (5α-DHLNG) is an active metabolite of the progestin levonorgestrel which is formed by 5α-reductase. It has about one-third of the affinity of levonorgestrel for the progesterone receptor. In contrast to levonorgestrel, the compound has both progestogenic and antiprogestogenic activity, and hence has a selective progesterone receptor modulator-like profile of activity. This is analogous to the case of norethisterone and 5α-dihydronorethisterone. In addition to the progesterone receptor, 5α-DHLNG interacts with the androgen receptor. It has similar affinity for the androgen receptor relative to levonorgestrel (34.3% of that of metribolone for levonorgestrel and 38.0% of that of metribolone for 5α-DHLNG), and has androgenic effects similarly to levonorgestrel and testosterone. 5α-DHLNG is further transformed into 3α,5α- and 3β,5α-, which bind weakly to the estrogen receptor (0.4 to 2.4% of the of ) and have weak estrogenic activity. These metabol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5α-reductase
5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of 5α-reductase encoded by the genes SRD5A1, SRD5A2, and SRD5A3. 5α-Reductases catalyze the following generalized chemical reaction: :a 3-oxo-5α-steroid + acceptor a 3-oxo-Δ4-steroid + reduced acceptor Where a 3-oxo-5α-steroid and acceptor are substrates, and a corresponding 3-oxo-Δ4-steroid and the reduced acceptor are products. An instance of this generalized reaction that 5α-reductase type 2 catalyzes is: :dihydrotestosterone + NADP+ \rightleftharpoons testosterone + NADPH + H+ where dihydrotestosterone is the 3-oxo-5α-steroid, NADP+ is the acceptor and testosterone is the 3-oxo-Δ4-steroid and NADPH the reduced acceptor. Production and activity The enzyme is produced in many tissues in both males and females, in the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5α-Dihydronorethisterone
5α-Dihydronorethisterone (5α-DHNET, dihydronorethisterone, 17α-ethynyl-5α-dihydro-19-nortestosterone, or 17α-ethynyl-5α-estran-17β-ol-3-one) is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues. Unlike norethisterone which is purely progestogenic, 5α-DHNET has been found to possess both progestogenic and marked antiprogestogenic activity, showing a profile of progestogenic activity like that of a selective progesterone receptor modulator (SPRM). Moreover, the affinity of 5α-DHNET for the progesterone receptor (PR) is greatly reduced relative to that of norethisterone at only 25% of that of progesterone (versus 150% for norethisterone). 5α-DHNET shows higher affinity for the androgen receptor (AR) compared to norethisterone with approximately 27% of the affinity of the potent androgen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
19-Noretiocholanolone
19-Noretiocholanolone, also known as 5β-estran-3α-ol-17-one, is a metabolite of nandrolone (19-nortestosterone) and bolandione (19-norandrostenedione) that is formed by 5α-reductase. It is on the list of substances prohibited by the World Anti-Doping Agency since it is a detectable metabolite of nandrolone, an anabolic-androgenic steroid (AAS). Traces of 19-noretiocholanolone may be naturally present in human urine. Consumption of boar meat, liver, kidneys and heart have been found to increase urinary 19-noretiocholanolone output. See also * Etiocholanolone Etiocholanolone, also known as 5β-androsterone, as well as 3α-hydroxy-5β-androstan-17-one or etiocholan-3α-ol-17-one, is an etiocholane (5β-androstane) steroid as well as an endogenous 17-ketosteroid that is produced from the metabolism of ... * 19-Norandrosterone * 5α-Dihydronandrolone * 5α-Dihydronorethisterone References {{DEFAULTSORT:Noretiocholanolone, 19- 5α-Reduced steroid metabolites Secondary a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5α-reductase Inhibitor
5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of 5α-reductase encoded by the genes SRD5A1, SRD5A2, and SRD5A3. 5α-Reductases catalyze the following generalized chemical reaction: :a 3-oxo-5α-steroid + acceptor a 3-oxo-Δ4-steroid + reduced acceptor Where a 3-oxo-5α-steroid and acceptor are substrates, and a corresponding 3-oxo-Δ4-steroid and the reduced acceptor are products. An instance of this generalized reaction that 5α-reductase type 2 catalyzes is: :dihydrotestosterone + NADP+ \rightleftharpoons testosterone + NADPH + H+ where dihydrotestosterone is the 3-oxo-5α-steroid, NADP+ is the acceptor and testosterone is the 3-oxo-Δ4-steroid and NADPH the reduced acceptor. Production and activity The enzyme is produced in many tissues in both males and females, in the rep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prostate Cancer
Prostate cancer is cancer of the prostate. Prostate cancer is the second most common cancerous tumor worldwide and is the fifth leading cause of cancer-related mortality among men. The prostate is a gland in the male reproductive system that surrounds the urethra just below the bladder. It is located in the hypogastric region of the abdomen. To give an idea of where it is located, the bladder is superior to the prostate gland as shown in the image The rectum is posterior in perspective to the prostate gland and the ischial tuberosity of the pelvic bone is inferior. Only those who have male reproductive organs are able to get prostate cancer. Most prostate cancers are slow growing. Cancerous cells may spread to other areas of the body, particularly the bones and lymph nodes. It may initially cause no symptoms. In later stages, symptoms include pain or difficulty urinating, blood in the urine, or pain in the pelvis or back. Benign prostatic hyperplasia may produce similar symptoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5α-Dihydronorethandrolone
5α-Dihydronorethandrolone (5α-DHNED), also known as 17α-ethyl-4,5α-dihydro-19-nortestosterone or as 17α-ethyl-5α-estran-17β-ol-3-one, is an androgen/ anabolic steroid and a metabolite of norethandrolone (as well as of the prodrug ethylestrenol) formed by 5α-reductase. Analogously to nandrolone and its 5α-reduced metabolite 5α-dihydronandrolone, 5α-DHNED shows reduced affinity for the androgen receptor relative to norethandrolone. Its affinity for the androgen receptor is specifically about 64% of that of norethandrolone. See also * 5α-Dihydronormethandrone * 5α-Dihydronorethisterone * 5α-Dihydrolevonorgestrel 5α-Dihydrolevonorgestrel (5α-DHLNG) is an active metabolite of the progestin levonorgestrel which is formed by 5α-reductase. It has about one-third of the affinity of levonorgestrel for the progesterone receptor. In contrast to levonorgestrel ... * Ethylestradiol References {{DEFAULTSORT:Dihydronorethandrolone, 5α- 5α-Reduced steroid m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
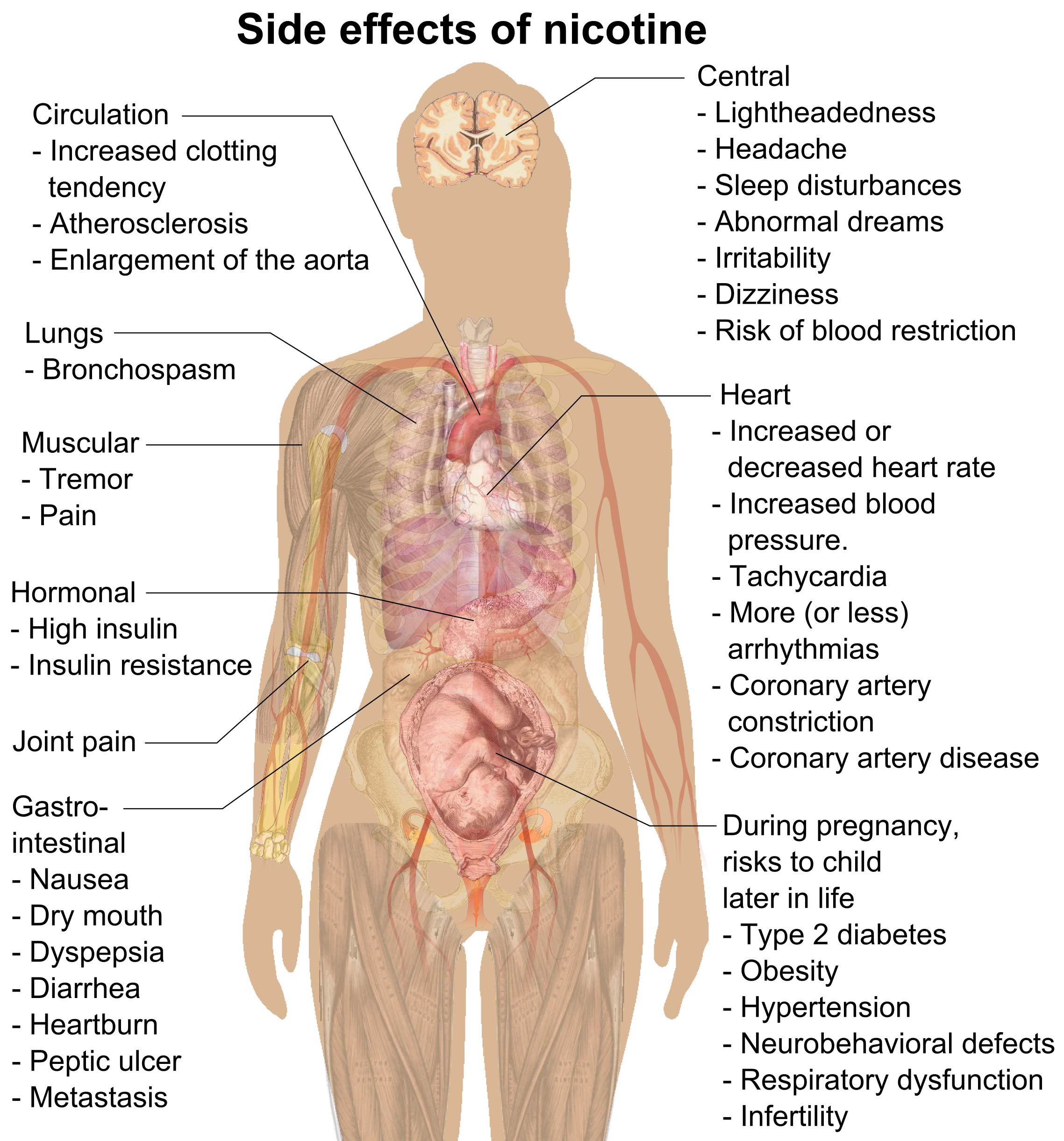
Side Effect
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequences of the use of a drug. Developing drugs is a complicated process, because no two people are exactly the same, so even drugs that have virtually no side effects, might be difficult for some people. Also, it is difficult to make a drug that targets one part of the body but that does not affect other parts, the fact that increases the risk of side effects in the untargeted parts. Occasionally, drugs are prescribed or procedures performed specifically for their side effects; in that case, said side effect ceases to be a side effect and is now an intended effect. For instance, X-rays were historically (and are currently) used as an imaging technique; the discovery of their oncolytic capability led to their employ in radiotherapy (ablation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dutasteride
Dutasteride, sold under the brand name Avodart among others, is a medication primarily used to treat the symptoms of a benign prostatic hyperplasia (BPH), an enlarged prostate not associated with cancer. A few months may be required before benefits occur. It is also used for scalp hair loss in men and as a part of hormone therapy in transgender women. It is usually taken by mouth. The most commonly reported side effects of dutasteride, although rare, include sexual dysfunction and depression. In the largest available study of 6,729 men with BPH, 9% experienced erectile dysfunction (compared to 5.7% treated with a placebo), 3.3% experienced decreased sex drive (vs 1.6% of placebo), and 1.9% had enlarged breasts (vs 1% of placebo). Exposure during pregnancy is specifically contraindicated because antiandrogens such as dutasteride have been shown to interfere with the sexual development of male fetuses. Dutasteride was patented in 1993 by GlaxoSmithKline and was approved for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Finasteride
Finasteride, sold under the brand names Proscar and Propecia among others, is a medication used to treat hair loss and benign prostatic hyperplasia (BPH) in men. It can also be used to treat excessive hair growth in women and as a part of hormone therapy for transgender women. It is taken by mouth. Finasteride is a 5α-reductase inhibitor and therefore an antiandrogen. It works by decreasing the production of dihydrotestosterone (DHT) by about 70%, including in the prostate gland and the scalp. In addition to DHT, finasteride also inhibits the production of several anticonvulsant neurosteroids including allopregnanolone, androstanediol, and THDOC. Adverse effects from finasteride are rare, however some men experience sexual dysfunction, depression, and breast enlargement. In some men, sexual dysfunction may persist after stopping the medication. It may also hide the early symptoms of certain forms of prostate cancer. Finasteride was patented in 1984 and approved for med ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |