|
5-hydroxyfuranocoumarin 5-O-methyltransferase
5-hydroxyfuranocoumarin 5-O-methyltransferase (, ''furanocoumarin 5-methyltransferase'', ''furanocoumarin 5-O-methyltransferase'', ''bergaptol 5-O-methyltransferase'', ''bergaptol O-methyltransferase'', ''bergaptol methyltransferase'', ''S-adenosyl-L-methionine:bergaptol O-methyltransferase'', ''BMT'', ''S-adenosyl-L-methionine:5-hydroxyfuranocoumarin 5.1-O-methyltransferase'') is an enzyme with systematic name ''S-adenosyl-L-methionine:5-hydroxyfurocoumarin 5-O-methyltransferase''. This enzyme catalyses the following chemical reaction : (1) S-adenosyl-L-methionine + a 5-hydroxyfurocoumarin \rightleftharpoons S-adenosyl-L-homocysteine + a 5-methoxyfurocoumarin (general reaction) : (2) S-adenosyl-L-methionine + bergaptol \rightleftharpoons S-adenosyl-L-homocysteine + bergapten Bergapten (5-methoxypsoralen) is a naturally-occurring organic chemical compound produced by numerous plant species, especially from the carrot family Apiaceae and the citrus family Rutaceae. For example, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Enzymes
This article lists enzymes by their classification in the International Union of Biochemistry and Molecular Biology's Enzyme Commission (EC) numbering system. * List of EC numbers (EC 5) * List of EC numbers (EC 6) :Oxidoreductases (EC 1) (Oxidoreductase) *Dehydrogenase * Luciferase *DMSO reductase :EC 1.1 (act on the CH-OH group of donors) * :EC 1.1.1 (with NAD+ or NADP+ as acceptor) ** Alcohol dehydrogenase (NAD) ** Alcohol dehydrogenase (NADP) **Homoserine dehydrogenase ** Aminopropanol oxidoreductase **Diacetyl reductase **Glycerol dehydrogenase **Propanediol-phosphate dehydrogenase ** glycerol-3-phosphate dehydrogenase (NAD+) ** D-xylulose reductase **L-xylulose reductase **Lactate dehydrogenase **Malate dehydrogenase **Isocitrate dehydrogenase ** HMG-CoA reductase * :EC 1.1.2 (with a cytochrome as acceptor) * :EC 1.1.3 (with oxygen as acceptor) **Glucose oxidase **L-gulonolactone oxidase **Thiamine oxidase **Xanthine oxidase * :EC 1.1.4 (with a disul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
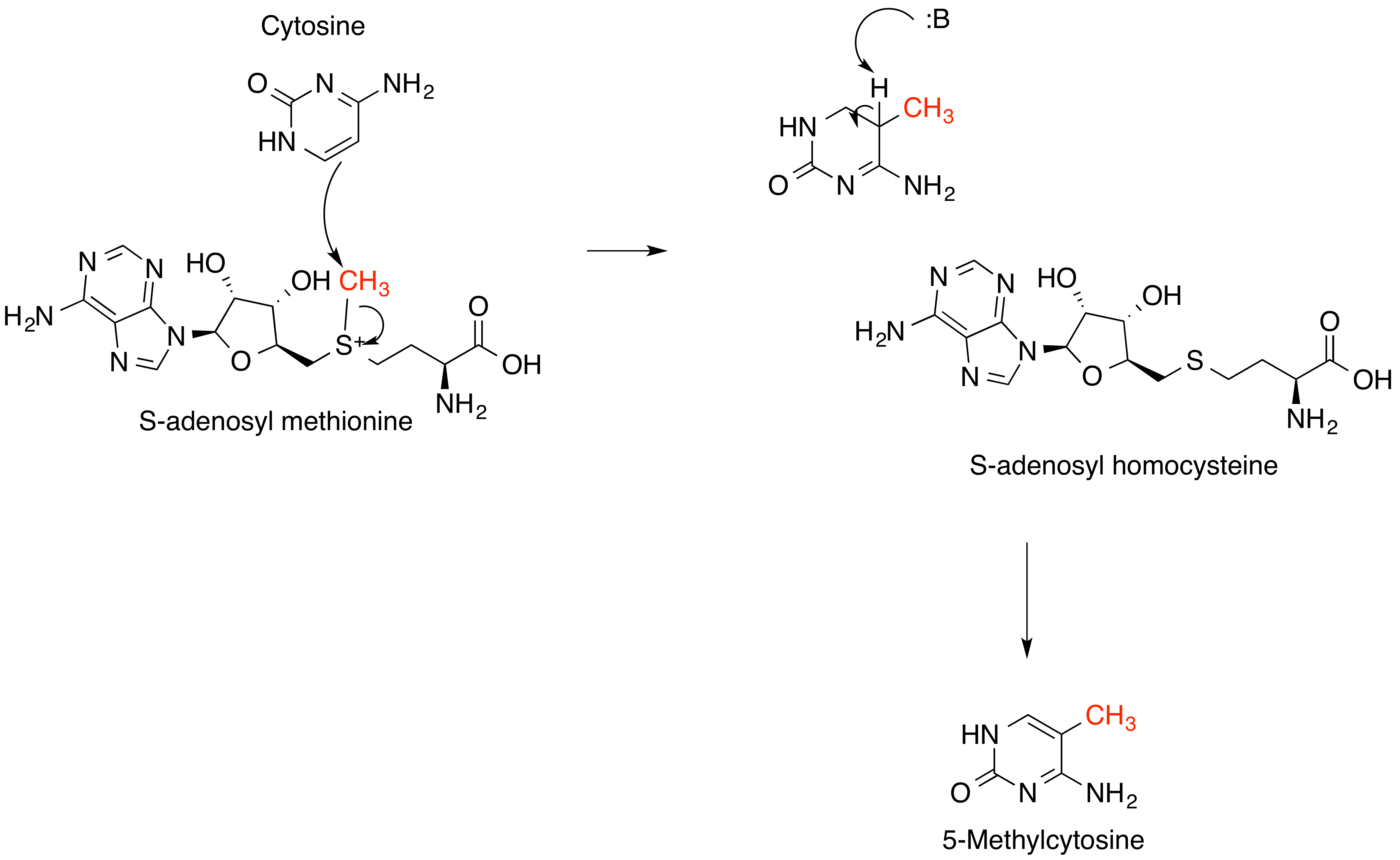
S-adenosyl-L-methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bergaptol
Bergaptol is a natural furanocoumarin with the molecular formula C11H6O4. It is found in the essential oils of citrus including lemon The lemon (''Citrus limon'') is a species of small evergreen trees in the flowering plant family Rutaceae, native to Asia, primarily Northeast India (Assam), Northern Myanmar or China. The tree's ellipsoidal yellow fruit is used for culin ... and bergamot. Notes Furanocoumarins Phenols {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bergapten
Bergapten (5-methoxypsoralen) is a naturally-occurring organic chemical compound produced by numerous plant species, especially from the carrot family Apiaceae and the citrus family Rutaceae. For example, bergapten has been extracted from 24 species of the genus '' Heracleum'' in the family Apiaceae. Cited by Mitchell and Rook (1979). In the family Rutaceae, various ''Citrus'' species contain significant amounts of bergapten, especially the bergamot orange, the micrantha, and certain varieties of lime and bitter orange. Bergapten belongs to a class of chemical compounds known as the furanocoumarins. In 1834, Kalbrunner isolated 5-methoxypsoralen from bergamot essential oil, hence the common name "bergapten". It was the first furanocoumarin to be isolated and identified. Toxicity Bergapten is a derivative of psoralen, the parent compound of a family of naturally-occurring organic compounds known as the linear furanocoumarins (so called since they exhibit a linear chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylcoumarins
Methylcoumarin is one of a series of isomers in which a methyl group substitutes for a hydrogen atom in coumarin Coumarin () or 2''H''-chromen-2-one is an aromatic organic chemical compound with formula . Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by a lactone-like chain , forming a second six-membered h .... They all have formula C10H8O2 and molecular weight 160.172 g/mol. References {{Reflist Coumarins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coumarins Metabolism
Coumarin () or 2''H''-chromen-2-one is an aromatic organic chemical compound with formula . Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by a lactone-like chain , forming a second six-membered heterocycle that shares two carbons with the benzene ring. It can be placed in the benzopyrone chemical class and considered as a lactone. Coumarin is a colorless crystalline solid with a sweet odor resembling the scent of vanilla and a bitter taste. It is found in many plants, where it may serve as a chemical defense against predators. By inhibiting synthesis of vitamin K, a related compound is used as the prescription drug warfarin – an anticoagulant – to inhibit formation of blood clots, deep vein thrombosis, and pulmonary embolism. Etymology Coumarin is derived from ''coumarou'', the French word for the tonka bean. The word ''tonka'' for the tonka bean is taken from the Galibi (Carib) tongue spoken by natives of French G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |