|
5-Formamidoimidazole-4-carboxamide Ribotide
5-Formamidoimidazole-4-carboxamide ribotide (or FAICAR) is an intermediate in the formation of purines. It is formed by the enzyme AICAR transformylase In enzymology, a phosphoribosylaminoimidazolecarboxamide formyltransferase (), also known by the shorter name AICAR transformylase, is an enzyme that catalyzes the chemical reaction :10-formyltetrahydrofolate + AICAR \rightleftharpoons tetrahyd ... from AICAR and 10-formyltetrahydrofolate. References Imidazoles Nucleotides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
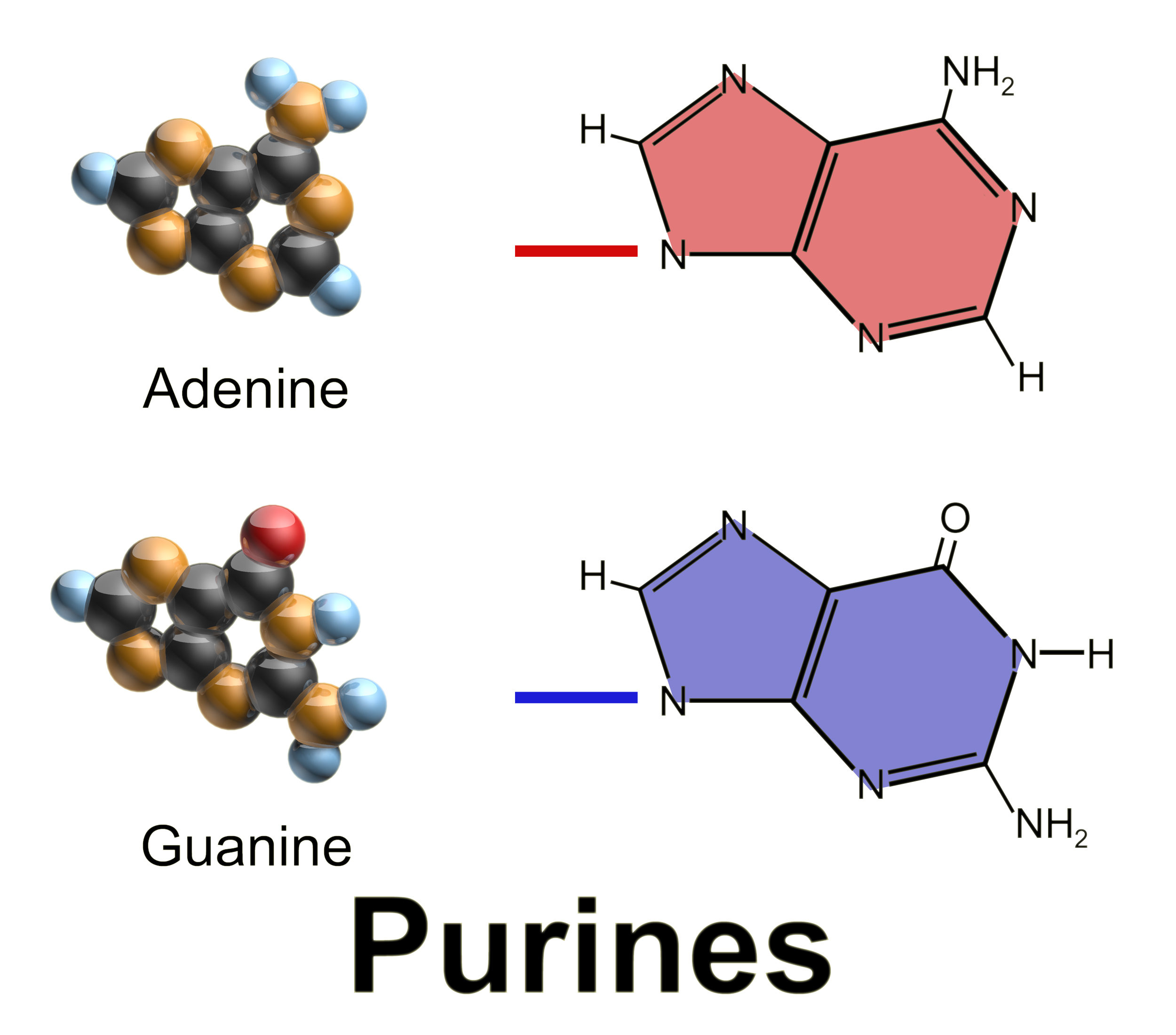
Purine
Purine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and Black-eyed pea, black eye peas) and Spirulina (dietary supplement), spirulina. Examples of high-purine sources include: sweetbreads, Anchovies as food, anchovies, Sardines as food, sardines, liver, beef kidneys, Brain as food, brains, meat extracts (e.g., Oxo (food), Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AICAR Transformylase
In enzymology, a phosphoribosylaminoimidazolecarboxamide formyltransferase (), also known by the shorter name AICAR transformylase, is an enzyme that catalyzes the chemical reaction :10-formyltetrahydrofolate + AICAR \rightleftharpoons tetrahydrofolate + FAICAR Thus, the two substrates of this enzyme are 10-formyltetrahydrofolate and AICAR, whereas its two products are tetrahydrofolate and FAICAR. This enzyme participates in purine metabolism and one carbon pool by folate. Nomenclature This enzyme belongs to the family of transferases that transfer one-carbon groups, specifically the hydroxymethyl-, formyl- and related transferases. The systematic name of this enzyme class is 10-formyltetrahydrofolate:5-phosphoribosyl-5-amino-4-imidazole-carb oxamide N-formyltransferase. Other names in common use include: * 10-formyltetrahydrofolate:5-phosphoribosyl-5-amino-4-imidazolecarboxamide formyltransferase * 5-amino-1-ribosyl-4-imidazolecarboxamide 5-phosphate, * 5-amino-4-imi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AICA Ribonucleotide
5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is an intermediate in the generation of inosine monophosphate. AICAR is an analog of adenosine monophosphate (AMP) that is capable of stimulating AMP-dependent protein kinase (AMPK) activity. The drug has also been shown as a potential treatment for diabetes by increasing the metabolic activity of tissues by changing the physical composition of muscle. Mechanism of action The nucleoside form of AICAR, acadesine, is an analog of adenosine that enters cardiac cells to inhibit adenosine kinase and adenosine deaminase. It enhances the rate of nucleotide re-synthesis increasing adenosine generation from adenosine monophosphate only during conditions of myocardial ischemia. In cardiac myocytes, acadesine is phosphorylated to AICAR to activate AMPK without changing the levels of the nucleotides. AICAR is able to enter the de novo synthesis pathway for adenosine synthesis to inhibit adenosine deaminase causing an increase in ATP level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazoles
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membered ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |