|
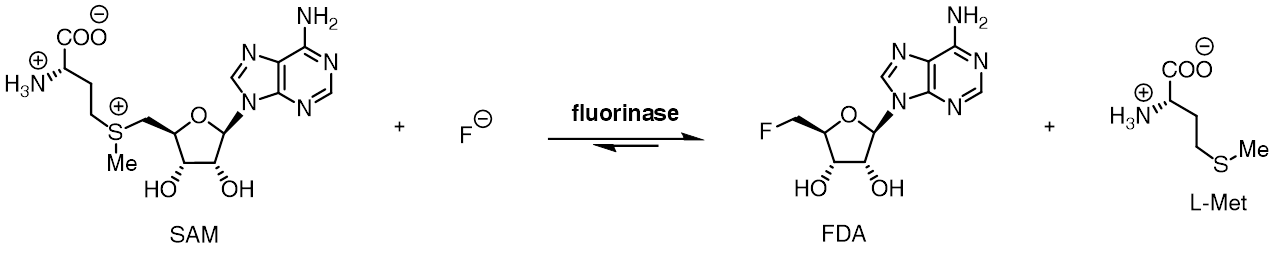
5'-deoxy-5'-fluoroadenosine
5′-Deoxy-5′-fluoroadenosine is the first step in the biosynthesis of organic fluorides. It is synthesized by the fluorinase catalyzed addition of a fluoride ion to ''S''-adenosyl-L-methionine, releasing L-methionine as a by product. Purine nucleoside phosphorylase Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase () is an enzyme that in humans is encoded by the ''NP'' gene. It catalyzes the chemical reaction :purine nucleoside + phosphate \rightleftharpoons purine + alpha-D-ribose 1- ... mediates a phosphorolytic cleavage of the adenine base to generate 5-fluoro-5-deoxy-D-ribose-1-phosphate. References {{DEFAULTSORT:Deoxy-5'-fluoroadenosine, 5- Organofluorides Nucleosides Fluorine-containing natural products ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Fluoro-5-deoxy-D-ribose-1-phosphate
5-Fluoro-5-deoxy--ribose 1-phosphate is metabolite formed during the biosynthesis of organofluorides. It is formed by the purine nucleoside phosphorylase mediated phosphorolytic cleavage of 5'-deoxy-5'-fluoroadenosine. It is isomerized to 5-fluoro-5-deoxy-ribulose-1-phosphate which is then cleaved by an aldolase to release fluoroacetaldehyde Fluoroacetaldehyde is a metabolic precursor of both fluoroacetate and 4-fluorothreonine in ''Streptomyces cattleya ''Streptomyces cattleya'' is a Gram-positive bacterium which makes cephamycin, penicillin and thienamycin. The bacterium express .... References {{DEFAULTSORT:Fluoro-5-deoxy-D-ribose 1-phosphate, 5- Organofluorides Organophosphates Halogen-containing natural products Fluorine-containing natural products ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorides
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosyl-fluoride Synthase
The fluorinase enzyme (, also known as adenosyl-fluoride synthase) catalyzes the reaction between fluoride ion and the co-factor '' S'' -adenosyl-L-methionine to generate L-methionine and 5'-fluoro-5'-deoxyadenosine, the first committed product of the fluorometabolite biosynthesis pathway. The fluorinase was originally isolated from the soil bacterium ''Streptomyces cattleya'', but homologues have since been identified in a number of other bacterial species, including ''Streptomyces'' sp. MA37, '' Nocardia brasiliensis'' and ''Actinoplanes'' sp. N902-109. This is the only known enzyme capable of catalysing the formation of a carbon-fluorine bond, the strongest single bond in organic chemistry. A homologous chlorinase enzyme, which catalyses the same reaction with chloride rather than fluoride ion, has been isolated from ''Salinospora tropica'', from the biosynthetic pathway of salinosporamide A. Reactivity The fluorinase catalyses an SN2-type nucleophilic substitution at the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-Adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the protonated fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine Nucleoside Phosphorylase
Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase () is an enzyme that in humans is encoded by the ''NP'' gene. It catalyzes the chemical reaction :purine nucleoside + phosphate \rightleftharpoons purine + alpha-D-ribose 1-phosphate Thus, the two substrates of this enzyme are a purine nucleoside and phosphate, whereas its products are a purine and alpha-D-ribose 1-phosphate. Nomenclature This enzyme belongs to the family of glycosyltransferases, specifically the pentosyltransferases. The systematic name of this enzyme class is purine-nucleoside:phosphate ribosyltransferase. Other names in common use include: * inosine phosphorylase * PNPase * PUNPI * PUNPII * inosine-guanosine phosphorylase * nucleotide phosphatase * purine deoxynucleoside phosphorylase * purine deoxyribonucleoside phosphorylase * purine nucleoside phosphorylase * purine ribonucleoside phosphorylas This enzyme participates in 3 metabolic pathways: purine metabolism, pyrimidine met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleosides
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building-blocks of DNA and RNA. List of nucleosides and corresponding nucleobases The reason for 2 symbols, shorter and longer, is that the shorter ones are better for contexts where explicit disambiguation is superfluous (because context disambiguates) and the longer ones are for contexts where explicit disambiguation is judged to be needed or wise. For example, when discussing long nucleobase sequences in genomes, the CATG symbol system is much preferable to the Cyt-Ade-Thy-Gua symbol system (see '' N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |