|
4-Chloromercuribenzoic Acid
4-Chloromercuribenzoic acid (''p''-chloromercuribenzoic acid, PCMB) is an organomercury compound that is used as a protease inhibitor, especially in molecular biology applications. PCMB reacts with thiol groups in proteins and is therefore an inhibitor of enzymes that are dependent on thiol reactivity, including cysteine proteases such as papain and acetylcholinesterase Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme Enzymes () are proteins that a .... Because of this reactivity with thiols, PCMB is also used in titrimetric quantification of thiol groups in proteins. See also * 4-Aminophenylmercuric acetate References {{DEFAULTSORT:Chloromercuribenzoic acid, 4- Benzoic acids Organomercury compounds Protease inhibitors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomercury Compound
Organomercury refers to the group of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs. The toxicity of organomercury compounds presents both dangers and benefits. Dimethylmercury in particular, is notoriously toxic, but found use as an antifungal agent and insecticide. Merbromin and phenylmercuric borate are used as topical antiseptics, while nitromersol is used as a preservative for vaccines and antitoxins. Synthesis Organomercury compounds are generated by many methods, including the direct reaction of hydrocarbons and mercury(II) salts. In this regard, organomercury chemistry more closely resembles organopalladium chemistry and contrasts wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease Inhibitor (biology)
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases (enzymes that aid the breakdown of proteins). Many naturally occurring protease inhibitors are proteins. In medicine, ''protease inhibitor'' is often used interchangeably with alpha 1-antitrypsin (A1AT, which is abbreviated PI for this reason). A1AT is indeed the protease inhibitor most often involved in disease, namely in alpha-1 antitrypsin deficiency. Classification Protease inhibitors may be classified either by the type of protease they inhibit, or by their mechanism of action. In 2004 Rawlings and colleagues introduced a classification of protease inhibitors based on similarities detectable at the level of amino acid sequence. This classification initially identified 48 families of inhibitors that could be grouped into 26 related superfamily (or clans) by their structure. According to the MEROPS database there are now 81 families of inhibitors. These fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol Group
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine Protease
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad. Discovered by Gopal Chunder Roy in 1873, the first cysteine protease to be isolated and characterized was papain, obtained from ''Carica papaya''. Cysteine proteases are commonly encountered in fruits including the papaya, pineapple, fig and kiwifruit. The proportion of protease tends to be higher when the fruit is unripe. In fact, the latex of dozens of different plant families are known to contain cysteine proteases. Cysteine proteases are used as an ingredient in meat tenderizers. Classification The MEROPS protease classification system counts 14 superfamilies plus several currently unassigned families (as of 2013) each containing many families. Each superfamily uses the catalytic triad or dyad in a different protein fold and so represent convergent evolutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Papain
Papain, also known as papaya proteinase I, is a cysteine protease () enzyme present in papaya (''Carica papaya'') and mountain papaya (''Vasconcellea cundinamarcensis''). It is the namesake member of the papain-like protease family. It has wide ranging commercial applications in the leather, cosmetic, textiles, detergents, food and pharmaceutical industries. In the food industry, papain is used as an active ingredient in many commercial meat tenderizers. Papain family Papain belongs to a family of related proteins, known as the papain-like protease family, with a wide variety of activities, including endopeptidases, aminopeptidases, dipeptidyl peptidases and enzymes with both exo- and endopeptidase activity. Members of the papain family are widespread, found in Baculoviridae, baculoviruses, eubacteria, yeast, and practically all protozoa, plants and mammals. The proteins are typically lysosomal or secreted, and proteolytic cleavage of the propeptide is required for enzyme activa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
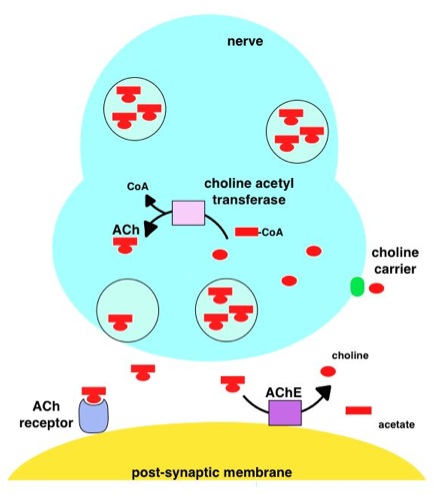
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ... that catalysis, catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate neurotransmission, synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' or ''titrator'', is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of ''analyte'' (which may also be termed the ''titrand'') to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the ''titration volume''. History and etymology The word "titration" descends from the French word ''titrer'' (1543), meaning the proportion of gold or silver in coins or in works of gold or silver; i.e., a measure of fineness or purity. ''Tiltre'' became ''titre'', which thus came to mean the "fineness of alloyed gold", and then the "concentration of a substance in a given sample". In 1828, the French chemist Joseph Louis Gay-Lussac first used ''titre'' as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Aminophenylmercuric Acetate
4-Aminophenylmercuric acetate (CH3CO2HgC6H4NH2, also known as 4-(Acetoxymercurio)aniline or APMA), is an organomercurial compound and thiol-blocking reagent used in experimental biology and chemistry to activate matrix metalloproteinases and collagenase proteolytic enzymes. The material is highly toxic. Properties APMA has a molecular weight of 351.8 g/mol and appears as a white powder with a slight yellowish cast. Its melting temperature is 163–165 °C. APMA is soluble in water to concentrations as high as 5 mM, and in DMSO to concentrations of 10 M or more. In 100% acetic acid, an APMA solution of 50 mg/mL is a light translucent yellow. Protein modification APMA is known to activate matrix metalloproteinase enzymes and collagenase. APMA activates proteolytic enzymes by reacting with cysteines at the amino terminal domains that bind zinc, near the location of the enzyme active site. Toxicity APMA and APMA vapors are highly toxic or fatal in contact with skin, or if inhaled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoic Acids
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates . History Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596). Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid. These latter also investigated how hippuric acid is related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomercury Compounds
Organomercury refers to the group of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs. The toxicity of organomercury compounds presents both dangers and benefits. Dimethylmercury in particular, is notoriously toxic, but found use as an antifungal agent and insecticide. Merbromin and phenylmercuric borate are used as topical antiseptics, while nitromersol is used as a preservative for vaccines and antitoxins. Synthesis Organomercury compounds are generated by many methods, including the direct reaction of hydrocarbons and mercury(II) salts. In this regard, organomercury chemistry more closely resembles organopalladium chemistry and contrasts wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |