|
4,4'-Azobis(4-cyanopentanoic Acid)
4,4′-Azobis(4-cyanopentanoic acid) (ACPA) is a free radical initiator used in polymer synthesis. ACPA is a water-soluble initiator used in both heterogeneous and homogeneous free-radical polymerizations. It is used as an initiator in reversible addition−fragmentation chain transfer polymerization (RAFT). When heated to decomposition Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is e ..., c. 70 °C, it releases N2 and produces 2 equivalents of reactive radicals capable of initiating polymerization. References Azo compounds Nitriles Dicarboxylic acids Radical initiators {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Initiator
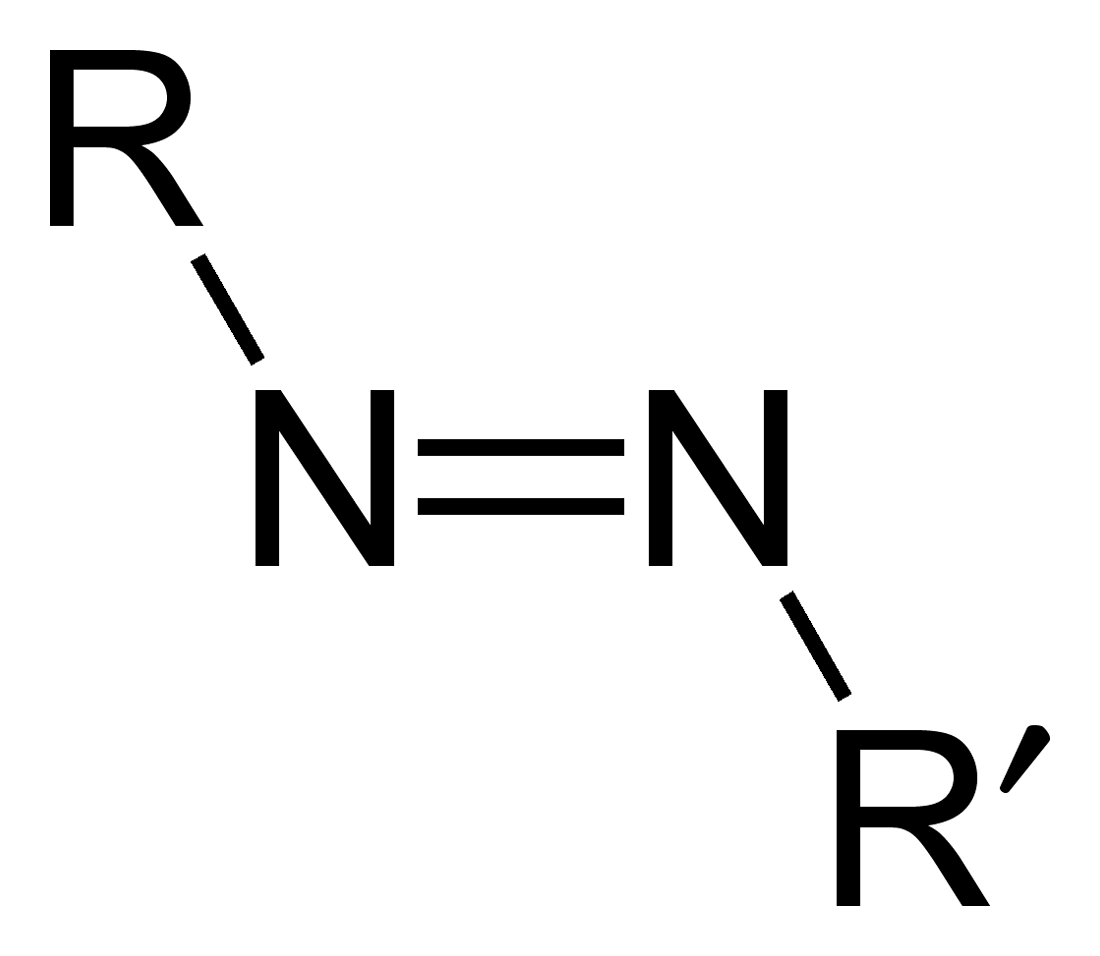
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such as polymer synthesis. Typical examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides. Main types of initiation reaction *Halogens undergo homolytic fission relatively easily. Chlorine, for example, gives two chlorine radicals (Cl•) by irradiation with ultraviolet light. This process is used for chlorination of alkanes. *Azo compounds (R- N=N-R') can be the precursor of two carbon-centered radicals (R• and R'•) and nitrogen gas upon heating and/or by irradiation. For example, AIBN and ABCN yield isobutyronitrile and cyclohexanecarbonitrile radicals, respectively. : *Organic peroxides each have a peroxide bond (- O-O-), which is readi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reversible Addition−fragmentation Chain Transfer Polymerization
Reversibility can refer to: * Time reversibility, a property of some mathematical or physical processes and systems for which time-reversed dynamics are well defined :* Reversible diffusion, an example of a reversible stochastic process * Reversible process (thermodynamics), a process or cycle such that the net change at each stage in the combined entropy of the system and its surroundings is zero * Reversible reaction A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously. : \mathit aA + \mathit bB \mathit cC + \mathit dD A and B can react to form C and D or, in the ..., a chemical reaction for which the position of the chemical equilibrium is very sensitive to the imposed physical conditions; so the reaction can be made to run either forwards or in reverse by changing those conditions * Reversible computing, logical reversibility of a computation; a computational step for which a we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make Calcium oxide, quick lime, which is an industrially impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitriles
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons. Inorganic compounds containing the group are not called nitriles, but cyanides instead. Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic. Structure and basic properties The N−C−C geometry is linear in nitriles, reflecting the sp hybridization of the triply bonded carbon. The C−N distance is short at 1.16 Å, consistent with a triple bond. Nitriles a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarboxylic Acids
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are used in the preparation of copolymers such as polyamides and polyesters. The most widely used dicarboxylic acid in the industry is adipic acid, which is a precursor in the production of nylon. Other examples of dicarboxylic acids include aspartic acid and glutamic acid, two amino acids in the human body. The name can be abbreviated to diacid. Linear saturated dicarboxylic acids The general formula is .Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. The PubChem links gives access to more information on the compounds, including other names, ids, toxicity and sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |