|
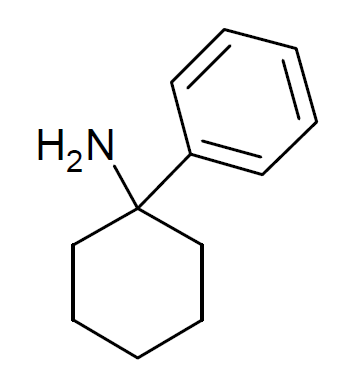
3'-MeO-PCP
3-Methoxyphencyclidine (3-MeO-PCP) is a dissociative hallucinogen of the arylcyclohexylamine class related to phencyclidine (PCP) which has been sold online as a designer drug. It acts mainly as an NMDA receptor antagonist, though it has also been found to interact with the sigma σ receptor and the serotonin transporter. The drug does not possess any opioid activity nor does it act as a dopamine reuptake inhibitor. Pharmacology 3-MeO-PCP has a K of 20 nM for the dizocilpine (MK-801) site of the NMDA receptor, 216 nM for the serotonin transporter (SERT), and 42 nM for the sigma σ receptor. It does not bind to the norepinephrine or dopamine transporter nor to the sigma σ receptor (Ki >10,000 nM). Based on its structural similarity to 3-hydroxy-PCP (3-HO-PCP), which uniquely among arylcyclohexylamines has high affinity for the μ-opioid receptor in addition to the NMDA receptor, it was initially expected that 3-MeO-PCP would have opioid activity. Howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylcyclohexylamine
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the class has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-hydroxy-PCP
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug. Pharmacology 3-HO-PCP acts as a high-affinity uncompetitive antagonist of the NMDA receptor via the dizocilpine (MK-801) site (Ki = 30 nM). It has much higher affinity than PCP for this site (Ki = 250 nM, for comparison; 8-fold difference). The drug also has high affinity for the μ-opioid receptor (MOR) (Ki = 39–60 nM) in animal test subjects, the κ-opioid receptor (KOR) (Ki = 140 nM), and the sigma σ1 receptor (Ki = 42 nM; IC50 = 19 nM), whereas it has only low affinity for the δ-opioid receptor (Ki = 2,300 nM). The high affinity of 3-HO-PCP for opioid receptors is unique among arylcyclohexylamines and is in contrast to PCP, which has only very low affinity for the MOR (Ki = 11,000–26,000 nM; 282- to 433-fold difference) and the other opioid receptors (Ki = 4,100&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United Kingdom
The United Kingdom of Great Britain and Northern Ireland, commonly known as the United Kingdom (UK) or Britain, is a country in Europe, off the north-western coast of the continental mainland. It comprises England, Scotland, Wales and Northern Ireland. The United Kingdom includes the island of Great Britain, the north-eastern part of the island of Ireland, and many smaller islands within the British Isles. Northern Ireland shares a land border with the Republic of Ireland; otherwise, the United Kingdom is surrounded by the Atlantic Ocean, the North Sea, the English Channel, the Celtic Sea and the Irish Sea. The total area of the United Kingdom is , with an estimated 2020 population of more than 67 million people. The United Kingdom has evolved from a series of annexations, unions and separations of constituent countries over several hundred years. The Treaty of Union between the Kingdom of England (which included Wales, annexed in 1542) and the Kingdom of Scotland in 170 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Advisory Council On The Misuse Of Drugs
The Advisory Council on the Misuse of Drugs (ACMD) is a British statutory advisory non-departmental public body, which was established under the Misuse of Drugs Act 1971. Mandate Its terms of reference, according to the Act, are as follows: ''to keep under review the situation in the United Kingdom with respect to drugs which are being or appear to them likely to be misused and of which the misuse is having or appears to them capable of having harmful effects sufficient to constitute a social problem, and to give to any one or more of the Ministers, where either Council consider it expedient to do so or they are consulted by the Minister or Ministers in question, advice on measures (whether or not involving alteration of the law) which in the opinion of the Council ought to be taken for preventing the misuse of such drugs or dealing with social problems connected with their misuse, and in particular on measures which in the opinion of the Council, ought to be taken'' *a) ''for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
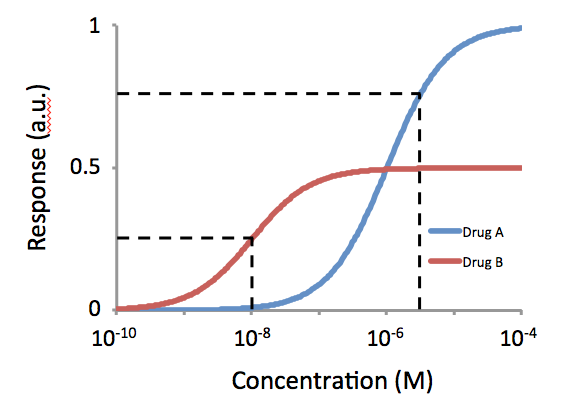
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Derivative
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structure–activity Relationship
The structure–activity relationship (SAR) is the relationship between the chemical structure of a molecule and its biological activity. This idea was first presented by Crum-Brown and Fraser in 1865. The analysis of SAR enables the determination of the chemical group responsible for evoking a target biological effect in the organism. This allows modification of the effect or the potency of a bioactive compound (typically a drug) by changing its chemical structure. Medicinal chemists use the techniques of chemical synthesis to insert new chemical groups into the biomedical compound and test the modifications for their biological effects. This method was refined to build mathematical relationships between the chemical structure and the biological activity, known as quantitative structure–activity relationships (QSAR). A related term is structure affinity relationship (SAFIR). Structure-biodegradability relationship The large number of synthetic organic chemicals currently in prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-MeO-PCP
4-Methoxyphencyclidine (methoxydine, 4-MeO-PCP) is a dissociative anesthetic drug that has been sold online as a research chemical. The synthesis of 4-MeO-PCP was first reported in 1965 by the Parke-Davis medicinal chemist Victor Maddox. A 1999 review published by a chemist using the pseudonym John Q. Beagle suggested the potency of 4-MeO-PCP in man was reduced relative to PCP, two years later Beagle published a detailed description of the synthesis and qualitative effects of 4-MeO-PCP, which he said possessed 70% the potency of PCP. 4-MeO-PCP was the first arylcyclohexylamine research chemical to be sold online, it was introduced in late 2008 by a company trading under the name CBAY and was followed by several related compounds such as 3-MeO-PCP and methoxetamine. 4-MeO-PCP has lower affinity for the NMDA receptor than PCP, but higher affinity than ketamine, it is orally active in a dosage range similar to ketamine, with some users requiring doses in excess of 100 mg for des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid Receptor
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract. Discovery By the mid-1960s, it had become apparent from pharmacologic studies that opiate drugs were likely to exert their actions at specific receptor sites, and that there were likely to be multiple such sites. Early studies had indicated that opiates appeared to accumulate in the brain. The receptors were first identified as specific molecules through the use of binding studies, in which opiates that had been labeled with radioisotopes were found to bind to brain membrane homogenates. The first such study was published in 1971, using 3H-levorphanol. In 1973, Candace Pert and Solomon H. Snyder publis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
κ-opioid Receptor
The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP, is a G protein-coupled receptor that in humans is encoded by the ''OPRK1'' gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction. The KOR is a type of opioid receptor that binds the opioid peptide dynorphin as the primary endogenous ligand (substrate naturally occurring in the body). In addition to dynorphin, a variety of natural alkaloids, terpenes and synthetic ligands bind to the receptor. The KOR may provide a natural addiction control mechanism, and therefore, drugs that target this receptor may have therapeutic potential in the treatment of addiction. There is evidence that distribution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |