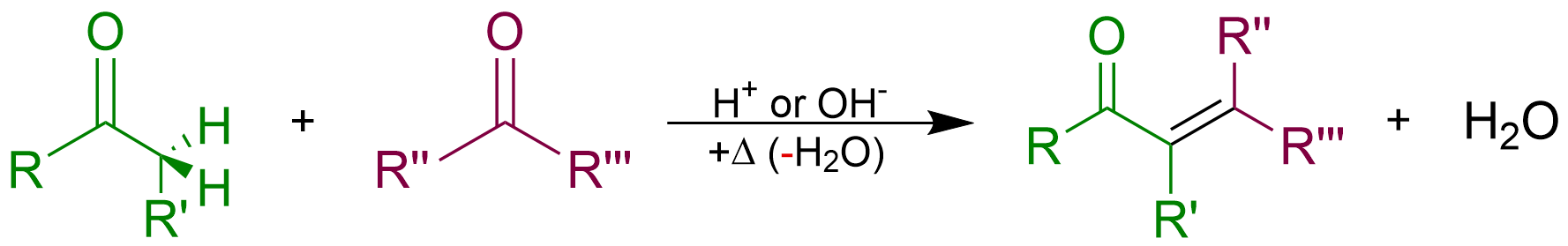|
3-deoxy-D-arabinoheptulosonate-7-phosphate
3-Deoxy--''arabino''-heptulosonic acid 7-phosphate (DAHP) is a 7-carbon ulonic acid. This compound is found in the shikimic acid biosynthesis pathway and is an intermediate in the production of aromatic amino acids. Phosphoenolpyruvate and erythrose-4-phosphate react to form 3-deoxy--''arabino''-heptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. : DAHP is then transformed to 3-dehydroquinate (DHQ), in a reaction catalyzed by DHQ synthase. Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD. : The mechanism of ring closure is complex, but involves an aldol condensation at C-2 and C-7. Metabolic engineering has improved production of DAHP by ''Escherichia coli ''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoenolpyruvate
Phosphoenolpyruvate (2-phosphoenolpyruvate, PEP) is the ester derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found (−61.9 kJ/mol) in organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system. In glycolysis PEP is formed by the action of the enzyme enolase on 2-phosphoglyceric acid. Metabolism of PEP to pyruvic acid by pyruvate kinase (PK) generates adenosine triphosphate (ATP) via substrate-level phosphorylation. ATP is one of the major currencies of chemical energy within cells. In gluconeogenesis PEP is formed from the decarboxylation of oxaloacetate and hydrolysis of one guanosine triphosphate molecule. This reaction is catalyzed by the enzyme pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythrose-4-phosphate
Erythrose 4-phosphate is a phosphate of the simple sugar erythrose. It is an intermediate in the pentose phosphate pathway and the Calvin cycle. In addition, it serves as a precursor in the biosynthesis of the aromatic amino acids tyrosine, phenylalanine, and tryptophan. It is used in the first step of the shikimate pathway. At this stage, phosphoenolpyruvate and erythrose-4-phosphate react to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. : It also used in 3-hydroxy-1-aminoacetone phosphate biosynthesis, which is a precursor of vitamin B6 in DXP-dependent pathway. Erythrose-4-phosphate dehydrogenase In enzymology, an erythrose-4-phosphate dehydrogenase () is an enzyme that catalyzes the chemical reaction :D-erythrose 4-phosphate + NAD+ + H2O \rightleftharpoons 4-phosphoerythronate + NADH + 2 H+ The 3 substrates of this enzyme are D-eryt ... is used to produce erythronate-4-phosphate. References {{bioc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DAHP Pathway , an intermediate in the biosynthesis of shikimic acid
{{disambiguation ...
DAHP may refer to: * Washington State Department of Archaeology and Historic Preservation, an independent government agency in Washington state * 3-deoxy-D-arabinoheptulosonate-7-phosphate 3-Deoxy--''arabino''-heptulosonic acid 7-phosphate (DAHP) is a 7-carbon ulonic acid. This compound is found in the shikimic acid biosynthesis pathway and is an intermediate in the production of aromatic amino acids. Phosphoenolpyruvate and er ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DAHP Synthase Reaction , an intermediate in the biosynthesis of shikimic acid
{{disambiguation ...
DAHP may refer to: * Washington State Department of Archaeology and Historic Preservation, an independent government agency in Washington state * 3-deoxy-D-arabinoheptulosonate-7-phosphate 3-Deoxy--''arabino''-heptulosonic acid 7-phosphate (DAHP) is a 7-carbon ulonic acid. This compound is found in the shikimic acid biosynthesis pathway and is an intermediate in the production of aromatic amino acids. Phosphoenolpyruvate and er ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous and easily confused with other types of drawing. The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers. Some notable uses include drawing sugars and depicting isomers. Conventions All bonds are depicted as horizontal or vertical lines. The carbon chain is depicted vertically, with carbon atoms sometimes not shown and represented by the center of crossing lines (see figure below). The orientation of the carbon chain is so that the first carbon (C1) is at the top. In an aldose, C1 is the carbon o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring Closure
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphates
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product physi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Engineering
Metabolic engineering is the practice of optimizing genetic and regulatory processes within cells to increase the cell's production of a certain substance. These processes are chemical networks that use a series of biochemical reactions and enzymes that allow cells to convert raw materials into molecules necessary for the cell's survival. Metabolic engineering specifically seeks to mathematically model these networks, calculate a yield of useful products, and pin point parts of the network that constrain the production of these products. Genetic engineering techniques can then be used to modify the network in order to relieve these constraints. Once again this modified network can be modeled to calculate the new product yield. The ultimate goal of metabolic engineering is to be able to use these organisms to produce valuable substances on an industrial scale in a cost-effective manner. Current examples include producing beer, wine, cheese, pharmaceuticals, and other biotechnolog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The cofactor is, therefore, found in two forms in cells: NAD is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |