|
3-Methoxymorphinan
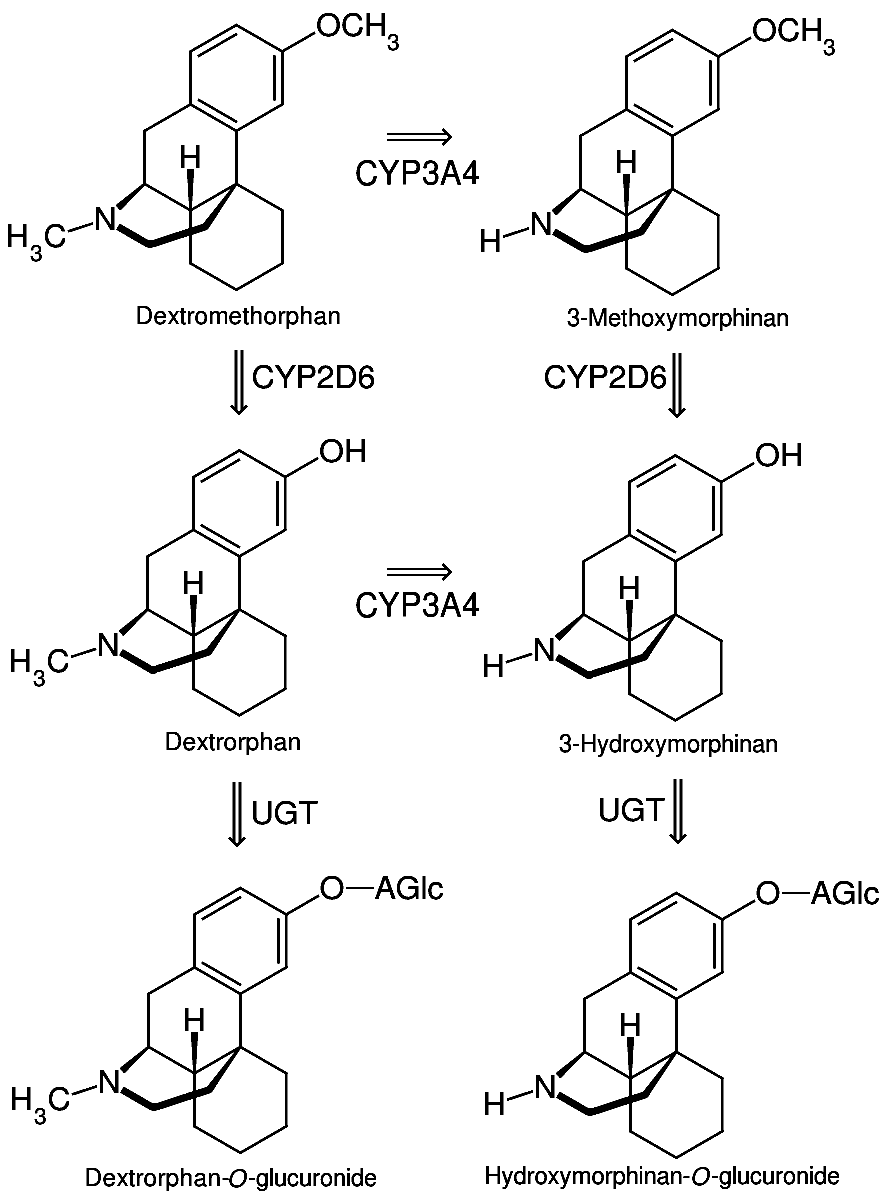
3-Methoxymorphinan is a levomethorphan metabolite that has been shown to produce local anesthetic effects. It is the CYP3A4 metabolite of dextromethorphan, and is itself metabolized by the liver enzyme CYP2D6. See also * 3-hydroxymorphinan * Dextrorphan * Dextromethorphan * Levomethorphan * Morphinan Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Structure Morphinan has a phenanthrene core stru ... References Morphinans Human drug metabolites {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphinan
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Structure Morphinan has a phenanthrene core structure with the ''A'' ring remaining aromatic and the ''B'' and ''C'' rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the ''D'' ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite. Of the major naturally occurring opiates of the morphinan type—morphine, codeine and thebaine—thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial production of at least four semi-synthetic opiate agonists, including hydrocodone, hydromorphone, oxycodone and oxymorphone, and the opioid antagonist naloxone. Structure-activity relationship The physiological behavior of morphinans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphinans
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Structure Morphinan has a phenanthrene core structure with the ''A'' ring remaining aromatic and the ''B'' and ''C'' rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the ''D'' ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite. Of the major naturally occurring opiates of the morphinan type—morphine, codeine and thebaine—thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial production of at least four semi-synthetic opiate agonists, including hydrocodone, hydromorphone, oxycodone and oxymorphone, and the opioid antagonist naloxone. Structure-activity relationship The physiological behavior of morphinans ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levomethorphan
Levomethorphan (LVM) ( INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of the racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine. Levomethorphan is a prodrug to levorphanol, analogously to DXM acting as a prodrug to dextrorphan or codeine behaving as a prodrug to morphine. As such, levomethorphan has similar effects to levorphanol but is less potent as it must be demethylated to the active form by liver enzymes before being able to produce its effects. As a prodrug of levorphanol, levomethorphan functions as a potent agonist of all three of the opioid receptors, μ, κ (κ1 and κ3 but notably not κ2), and δ, as an NMDA receptor antagonist, and as a serotonin-norepinephrine reuptake inhibitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levomethorphan
Levomethorphan (LVM) ( INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of the racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine. Levomethorphan is a prodrug to levorphanol, analogously to DXM acting as a prodrug to dextrorphan or codeine behaving as a prodrug to morphine. As such, levomethorphan has similar effects to levorphanol but is less potent as it must be demethylated to the active form by liver enzymes before being able to produce its effects. As a prodrug of levorphanol, levomethorphan functions as a potent agonist of all three of the opioid receptors, μ, κ (κ1 and κ3 but notably not κ2), and δ, as an NMDA receptor antagonist, and as a serotonin-norepinephrine reuptake inhibitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A4
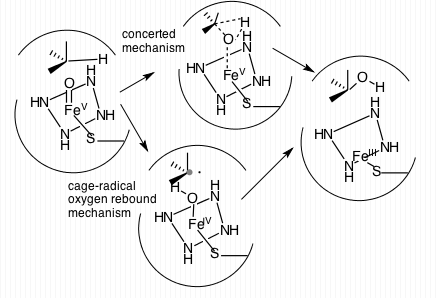
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs which are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will therefore either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprotein, i.e. a protein containing a heme group with an iron atom. In humans, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextromethorphan
Dextromethorphan (DXM) is a medication most often used as a cough suppressant in over-the-counter cold and cough medicines. It is sold in syrup, tablet, spray, and lozenge forms. In 2022, the FDA approved a formulation of it combined with bupropion named Auvelity to serve as a rapid acting antidepressant in patients with major depressive disorder. It is in the morphinan class of medications with sedative, dissociative, and stimulant properties (at lower doses). Dextromethorphan does not have a significant affinity for the mu-opioid receptor activity typical of morphinan compounds and exerts its therapeutic effects through several other receptors. In its pure form, dextromethorphan occurs as a white powder. Dextromethorphan is also used recreationally. When exceeding approved dosages, dextromethorphan acts as a dissociative hallucinogen. It has multiple mechanisms of action, including actions as a nonselective serotonin reuptake inhibitor and a sigma-1 receptor agonis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2D6
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the ''CYP2D6'' gene. ''CYP2D6'' is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra. CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation. CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as hydroxytryptamines, neurosteroids, and both ''m''-tyramine and ''p''-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver. Considerable variation exists in the efficiency and amount of CYP2D6 enzyme produced betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-hydroxymorphinan
3-Hydroxymorphinan (3-HM), or morphinan-3-ol, is a psychoactive drug of the morphinan family. It is the racemic counterpart to norlevorphanol. The dextrorotatory stereoisomer of the compound is an active metabolite of dextromethorphan, dextrorphan, and 3-methoxymorphinan, and similarly to them has potent neuroprotective and neurotrophic effects on LTS- and MPTP-treated dopaminergic neurons of the nigrostriatal pathway, but notably without producing any neuropsychotoxic side effects (e.g., dissociation or hallucinations) or having any anticonvulsant actions. It does not seem to bind to the NMDA receptor, and instead, its neuroprotective properties appear result from inhibition of glutamate release via the suppression of presynaptic voltage-dependent Ca2+ entry and protein kinase C activity. In any case, as such, the compound has been investigated as a potential management of Parkinson's disease medication (antiparkinsonian agent). A prodrug, GCC1290K, has been developed on ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextrorphan
Dextrorphan (DXO) is a psychoactive drug of the morphinan class which acts as an antitussive or cough suppressant and dissociative hallucinogen. It is the dextrorotatory enantiomer of racemorphan; the levorotatory enantiomer is levorphanol. Dextrorphan is produced by O-demethylation of dextromethorphan by CYP2D6. Dextrorphan is an NMDA antagonist and contributes to the psychoactive effects of dextromethorphan. Pharmacology Pharmacodynamics The pharmacology of dextrorphan is similar to that of dextromethorphan (DXM). However, dextrorphan is much more potent as an NMDA receptor antagonist as well much less active as a serotonin reuptake inhibitor, but retains DXM's activity as a norepinephrine reuptake inhibitor. It also has more affinity for the opiod receptors than dextromethorphan, significantly so at high doses. Pharmacokinetics Dextrorphan has a notably longer elimination half-life than its parent compound, and therefore has a tendency to accumulate in the blood after repe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextromethorphan
Dextromethorphan (DXM) is a medication most often used as a cough suppressant in over-the-counter cold and cough medicines. It is sold in syrup, tablet, spray, and lozenge forms. In 2022, the FDA approved a formulation of it combined with bupropion named Auvelity to serve as a rapid acting antidepressant in patients with major depressive disorder. It is in the morphinan class of medications with sedative, dissociative, and stimulant properties (at lower doses). Dextromethorphan does not have a significant affinity for the mu-opioid receptor activity typical of morphinan compounds and exerts its therapeutic effects through several other receptors. In its pure form, dextromethorphan occurs as a white powder. Dextromethorphan is also used recreationally. When exceeding approved dosages, dextromethorphan acts as a dissociative hallucinogen. It has multiple mechanisms of action, including actions as a nonselective serotonin reuptake inhibitor and a sigma-1 receptor agonis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-3-Hydroxymorphinan.png)