|
3,3',5,5'-tetramethylbenzidine
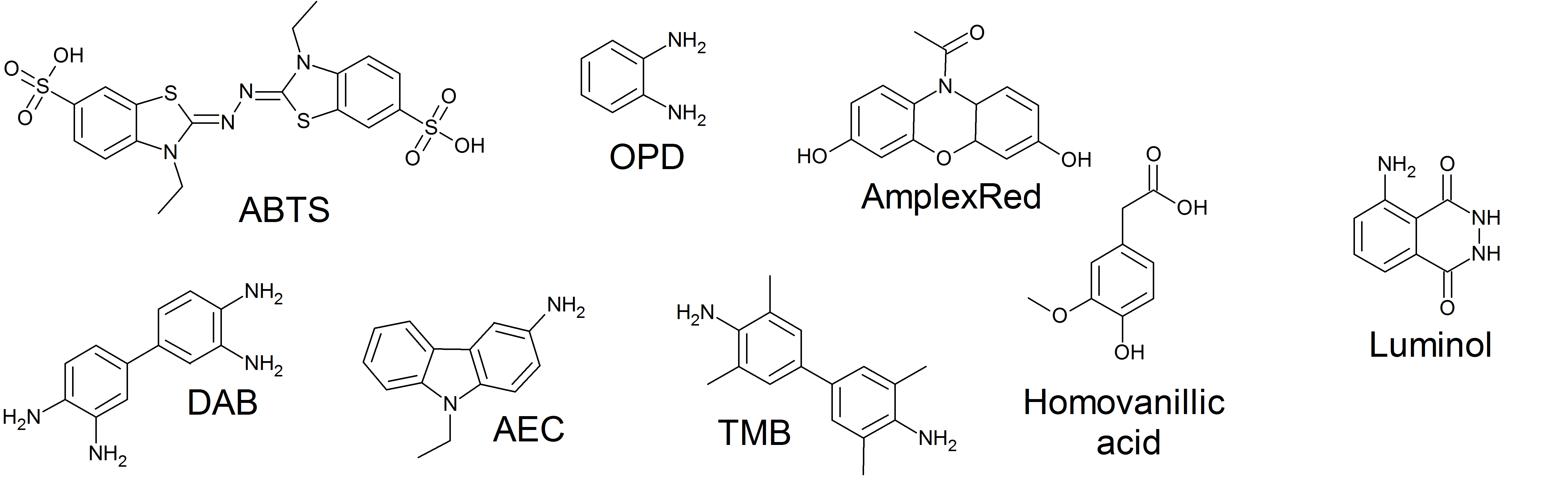
3,3′,5,5′-Tetramethylbenzidine or TMB is a chromogenic substrate used in staining procedures in immunohistochemistry as well as being a visualising reagent used in enzyme-linked immunosorbent assays (ELISA). TMB is a white solid that forms a pale blue-green liquid in solution with ethyl acetate. TMB is degraded by sunlight and by fluorescent lights. Enzymatic assay TMB can act as a hydrogen donor for the reduction of hydrogen peroxide to water by peroxidase enzymes such as horseradish peroxidase. The resulting one-electron oxidation product is a diimine-diamine complex, which causes the solution to take on a blue colour, and this colour change can be read on a spectrophotometer at the wavelengths of 370 and 650 nm. The reaction can be halted by addition of acid or another stop reagent. Using sulfuric acid Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Horseradish Peroxidase
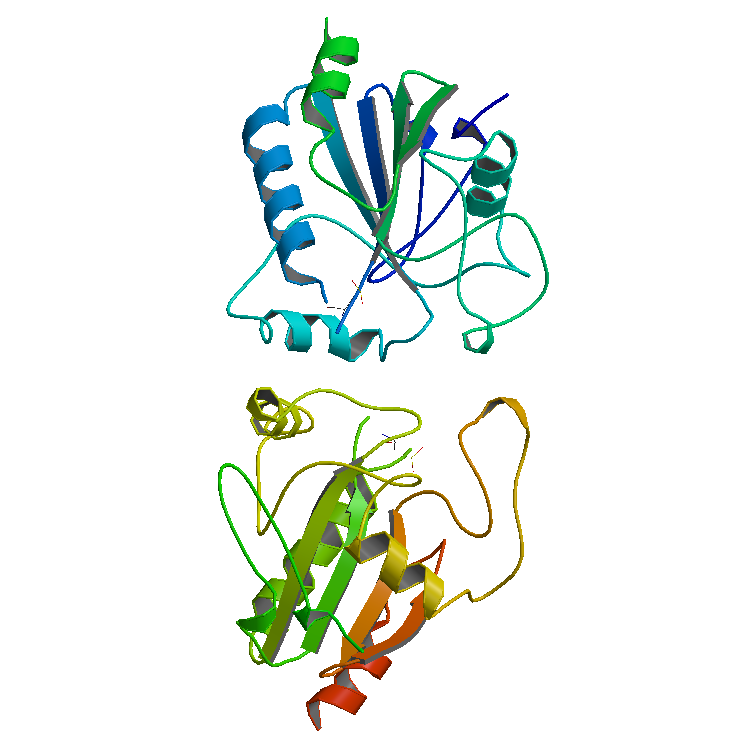
The enzyme horseradish peroxidase (HRP), found in the roots of horseradish, is used extensively in biochemistry applications. It is a metalloenzyme with many isoforms, of which the most studied type is C. It catalyzes the oxidation of various organic substrates by hydrogen peroxide. Structure The structure of the enzyme was first solved by X-ray crystallography in 1997; and has since been solved several times with various substrates. It is a large alpha-helical glycoprotein which binds heme as a redox cofactor. Substrates Alone, the HRP enzyme, or conjugates thereof, is of little value; its presence must be made visible using a substrate that, when oxidized by HRP using hydrogen peroxide as the oxidizing agent, yields a characteristic color change that is detectable by spectrophotometric methods. Numerous substrates for horseradish peroxidase have been described and commercialized to exploit the desirable features of HRP. These substrates fall into several distinct cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand (commonly a protein) in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries. In the most simple form of an ELISA, antigens from the sample to be tested are attached to a surface. Then, a matching antibody is applied over the surface so it can bind the antigen. This antibody is linked to an enzyme and then any unbound antibodies are removed. In the final step, a substance containing the enzyme's substrate is added. If there was binding, the subsequent reaction produces a detectable signal, most commonly a color change. Performing an ELISA involves at least ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunohistochemistry
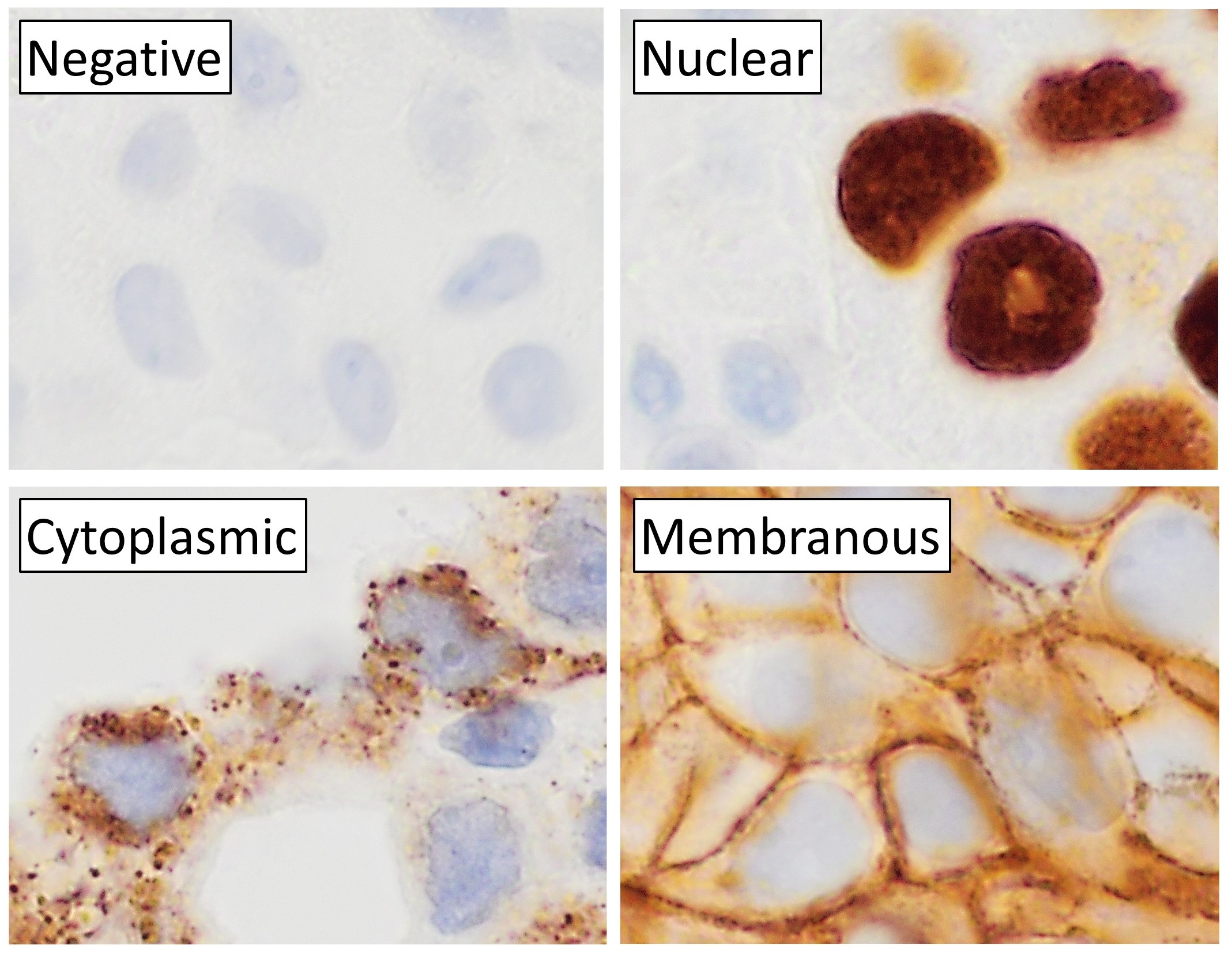
Immunohistochemistry (IHC) is the most common application of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue (compare to immunocytochemistry). Albert Coons conceptualized and first implemented the procedure in 1941. Visualising an antibody-antigen interaction can be accomplished in a number of ways, mainly either of the following: * ''Chromogenic immunohistochemistry'' (CIH), wherein an antibody is conjugated to an enzyme, such as peroxidase (the combination being termed immunoperoxidase), that can catalyse a colour-producing reaction. * '' Immunofluorescence'', where the antibody is tagged to a fluorophore, such as fluorescein or rhodamine. Immunohistochemical staining is widely used in the dia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. Production and synthesis Ethyl acetate was first synthesized by the Count de Lauraguais in 1759 by distilling a mixture of ethanol and acetic acid. In 2004, an estimated 1.3 million tonnes were produced worldwide. The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tonnes. The global ethyl acetate market was valued at $3.3 billion in 2018. Ethyl acetate is synthesized in industry mainly via the classic Fischer esterification reaction of ethanol and acetic acid. This mixture converts to the ester in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Lights
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the lamp to glow. A fluorescent lamp converts electrical energy into useful light much more efficiently than an incandescent lamp. The typical luminous efficacy of fluorescent lighting systems is 50–100 lumens per watt, several times the efficacy of incandescent bulbs with comparable light output. For comparison, the luminous efficacy of an incandescent bulb may only be 16 lumens per watt. Fluorescent lamp fixtures are more costly than incandescent lamps because, among other things, they require a ballast to regulate current through the lamp, but the initial cost is offset by a much lower running cost. Compact fluorescent lamps are now available in the same popular sizes as incand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides. Functionality Peroxidases typically catalyze a reaction of the form: :ROOR' + \overset + 2H+ -> ce + R'OH Optimal substrates For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides. Peroxidases can contain a heme cofactor in their active sites, or alternately redox-active cysteine or selenocysteine residues. The nature of the electron donor is very dependent on the structure of the enzyme. * For example, horseradish peroxidase can use a variety of organic compounds as electron donors and acceptors. Horseradish peroxidase has an accessible active site, and many compounds can reach the site of the reaction. * On the other hand, for an enzyme such as cytochrome c peroxidase, the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diimine
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated. Similar methods are used to prepare Schiff bases and oximes. 1,2-Diimines The 1,2-diketimine ligands also called α-diimines and 1,4-diazabutadienes. They are derived from the condensation of 1,2-diketones and glyoxal with amines, often anilines. An example is glyoxal-bis(mesitylimine), a yellow solid that is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal. 2,2'-Bipyridine is a 1,2-diimine. 1,2-Diketimines are “non-innocent ligands”, akin to the dithiolenes. : 1,3-Diimines For example, acetylacetone (2,4-pentanedione) and a primary alkyl- or arylamine will react, typically in acidified ethanol, to form a diketimine. 1,3-Diketimi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)
