|
2,2'-Biphenol
2,2′-Biphenol is an organic compound with the formula (C6H4OH)2. It is one of three symmetrical isomers of biphenol. A white solid, it is a precursor to diphosphite ligands that are used to support industrial hydroformylation catalysis. 244px, left, BiPhePhos is representative diphosphite ligand derived from 2,2′-biphenol. Synthesis Ring-opening of Dibenzofuran Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of t ... affords 2,2′-biphenol. Alternatively, it can be produced from 2,4-di-''tert''-butylphenol in two steps. The first step entails oxidative coupling to give the 2,2′-biphenol with four ''tert''-Bu substituents. This species then undergoes debutylation. See also * 1,1′-Bi-2-naphthol References {{DEFAULTSORT:Biphenol, 2, 2'- Phenols Biphenyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biphenol
In organic chemistry, a biphenol refers to compounds with the formula (C6H4OH)2. Such compounds formally result from the coupling of two phenols. {{short description, Chemical compound Three symmetrical isomer In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Iso ...s of biphenol exist: * 2,2'-Biphenol ( RN 1806-29-7) m.p. 109 °C * 3,3'-Biphenol (RN 612-76-0) m.p. 124.8 °C * 4,4'-Biphenol (RN 92-88-6) m.p. 283 °C Additionally, three unsymmetrical isomers of biphenol exist: * 2,3'-Biphenol (RN 31835-45-7) * 2,4'-Biphenol (RN 611-62-1) m.p. 162-163 °C * 3,4'-Biphenol (RN 18855-13-5) m.p. 190 °C Phenols Biphenyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphite Ligand
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivatives: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroformylation Catalysis
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: Production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and drugs. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry. The process entails treatment of an alkene typically with high pressures (between 10 and 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. In one variation, formaldehyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
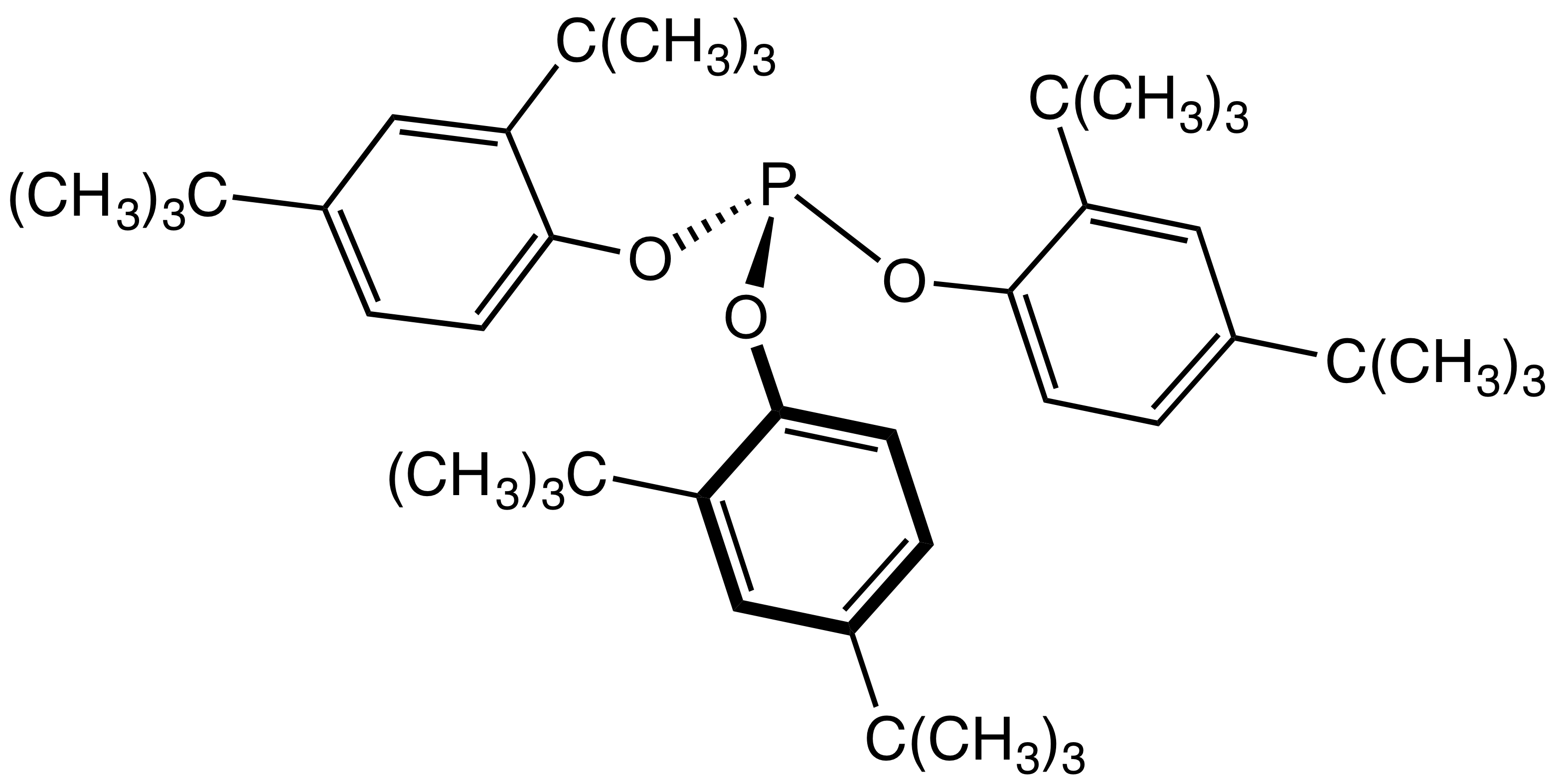
BiPhePhos
BiPhePhos is an organophosphorus compound that is used as a ligand in homogeneous catalysis. Classified as a diphosphite, BiPhePhos is derived from three 2,2'-biphenol groups, which constrain its shape in such a way to confer high selectivity to derived catalysts. Originally described by workers at Union Carbide Union Carbide Corporation is an American chemical corporation wholly owned subsidiary (since February 6, 2001) by Dow Chemical Company. Union Carbide produces chemicals and polymers that undergo one or more further conversions by customers befor ..., it has become a standard ligand in hydroformylation.{{cite journal, doi=10.1021/OM950549K, title=Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization, journal=Organometallics, volume=15, issue=2, pages=835–847, year=1996, last1=Van Rooy, first1=Annemiek, last2=Kamer, first2=Paul C. J., last3=Van Leeuwen, first3=Piet W. N. M., last4=Goubitz, first4=Kees, last5=Fraanje, first5=Jan, last6=Veldm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzofuran
Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.Gerd Collin and Hartmut Höke "Benzofurans" in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. Reactions Dibenzofuran is thermally robust with a convenient liquid range. These properties, together with its low toxicity, are exploited by the use of DBF as a heat transfer agent. It undergoes electrophilic reactions, such as halogenation and Friedel-Crafts reactions. Reaction of DBF with butyl lithium results in dilithiation.Ulrich Iserloh, Yoji Oderaotoshi, Shuji Kanemasa, and Dennis P. Curran "Synthesis of (R,R)-4,6-Dibenzofurandiyl-2,2'-Bis (4-Phenyloxazoline) (DBFO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1′-Bi-2-naphthol
1,1′-Bi-2-naphthol (BINOL) is an organic compound that is often used as a ligand for transition-metal catalysed asymmetric synthesis. BINOL has axial chirality and the two enantiomers can be readily separated and are stable toward racemisation. The specific rotation of the two enantiomers is 35.5° (''c'' = 1 in THF), with the ''R'' enantiomer being the dextrorotary one. BINOL is a precursor for another chiral ligand called BINAP. The volumetric mass density of the two enantiomers is 0.62 g cm. Preparation The organic synthesis of BINOL is not a challenge as such but the preparation of the individual enantiomers is. (''S'')-BINOL can be prepared directly from an asymmetric oxidative coupling of 2-naphthol with copper(II) chloride. The chiral ligand in this reaction is (''S'')-(+)-amphetamine. Racemic BINOL can also be produced using iron(III) chloride as an oxidant. The mechanism involves complexation of iron(III) into the hydroxyl, followed by a radical coupling react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |