|
2'-5'-oligoadenylate Synthase
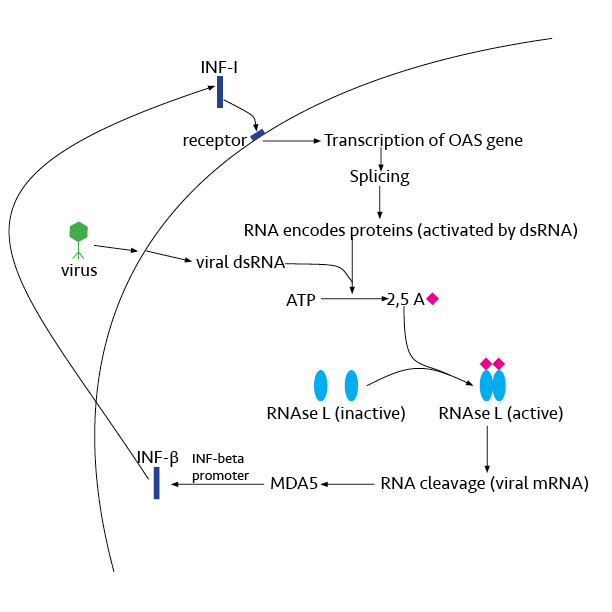
In molecular biology, 2'-5'-oligoadenylate synthetase (2-5A synthetase) is an enzyme () that reacts to interferon signal. It is an antiviral enzyme that counteracts viral attack by degrading RNAs, both viral and host. The enzyme uses ATP in 2'-specific nucleotidyl transfer reactions to synthesize 2'-5'-oligoadenylates, which activate latent ribonuclease (RNASEL), resulting in degradation of viral RNA and inhibition of virus replication. The C-terminal half of 2'-5'-oligoadenylate synthetase, also referred to as domain 2 of the enzyme, is largely alpha-helical and homologous to a tandem ubiquitin repeat. It carries the region of enzymatic activity between at the extreme C-terminal end. Human proteins * OAS1 * OAS2 * OAS3 2'-5'-oligoadenylate synthetase 3 is an enzyme that in humans is encoded by the ''OAS3'' gene. This gene encodes an enzyme included in the 2', 5' oligoadenylate synthase family. This enzyme is induced by interferons and catalyzes the 2', 5' o ... See al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-helical
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OAS3
2'-5'-oligoadenylate synthetase 3 is an enzyme that in humans is encoded by the ''OAS3'' gene. This gene encodes an enzyme included in the 2', 5' oligoadenylate synthase family. This enzyme is induced by interferons and catalyzes the 2', 5' oligomers of ATP. These oligomers activate latent RNase L Ribonuclease L or RNase L (for ''latent''), known sometimes as ribonuclease 4 or 2'-5' oligoadenylate synthetase-dependent ribonuclease — is an interferon (IFN)-induced ribonuclease which, upon activation, destroys all RNA within the cell (bot ..., leading to degradation of both viral and endogenous RNA. This enzyme family plays a significant role in the inhibition of cellular protein synthesis in response to viral infection. References Further reading * * * * * * * * * External links * {{gene-12-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OAS2
2'-5'-oligoadenylate synthetase 2 is an enzyme that in humans is encoded by the ''OAS2'' gene. This gene encodes a member of the 2-5A synthetase family, essential proteins involved in the innate immune response to viral infection. The encoded protein is induced by interferons and uses adenosine triphosphate in 2'-specific nucleotidyl transfer reactions to synthesize 2',5'-oligoadenylates (2-5As). These molecules activate latent RNase L, which results in viral RNA degradation and the inhibition of viral replication. The three known members of this gene family are located in a cluster on chromosome 12. Alternatively spliced Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be ... transcript variants encoding different isoforms have been described. References Further reading * * * * * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OAS1
2'-5'-oligoadenylate synthetase 1 is an enzyme that in humans is encoded by the ''OAS1'' gene. This gene encodes a member of the 2-5A synthetase family, which include essential proteins involved in the innate immune response to viral infection. The encoded protein is induced by interferons and uses adenosine triphosphate in 2'-specific nucleotidyl transfer reactions to synthesize 2',5'-oligoadenylates (2-5As). These molecules activate latent RNase L, which results in both viral and endogenous RNA degradation and the inhibition of viral replication. The three known members of this gene family are located in a cluster on chromosome 12. Hypomorphic mutations in this gene have been associated with host susceptibility to viral infection, while gain-of-function variants can cause autoinflammatory Periodic fever syndromes are a set of disorders characterized by recurrent episodes of systemic and organ-specific inflammation. Unlike autoimmune disorders such as systemic lupus erythemat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tandem Repeat
Tandem repeats occur in DNA when a pattern of one or more nucleotides is repeated and the repetitions are directly adjacent to each other. Several protein domains also form tandem repeats within their amino acid primary structure, such as armadillo repeats. However, in proteins, perfect tandem repeats are unlikely in most ''in vivo'' proteins, and most known repeats are in proteins which have been designed. An example would be: : ATTCG ATTCG ATTCG in which the sequence ATTCG is repeated three times. Terminology When between 10 and 60 nucleotides are repeated, it is called a minisatellite. Those with fewer are known as microsatellites or short tandem repeats. When exactly two nucleotides are repeated, it is called a ''dinucleotide repeat'' (for example: ACACACAC...). The microsatellite instability in hereditary nonpolyposis colon cancer most commonly affects such regions. When three nucleotides are repeated, it is called a ''trinucleotide repeat'' (for example: CAGCAGCAGCAG. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A. The addition of ubiquitin to a substrate protein is called ubiquitylation (or, alternatively, ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology (biology)
In biology, homology is similarity due to shared ancestry between a pair of structures or genes in different taxa. A common example of homologous structures is the forelimbs of vertebrates, where the wings of bats and birds, the arms of primates, the front flippers of whales and the forelegs of four-legged vertebrates like dogs and crocodiles are all derived from the same ancestral tetrapod structure. Evolutionary biology explains homologous structures adapted to different purposes as the result of descent with modification from a common ancestor. The term was first applied to biology in a non-evolutionary context by the anatomist Richard Owen in 1843. Homology was later explained by Charles Darwin's theory of evolution in 1859, but had been observed before this, from Aristotle onwards, and it was explicitly analysed by Pierre Belon in 1555. In developmental biology, organs that developed in the embryo in the same manner and from similar origins, such as from matching p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interferon
Interferons (IFNs, ) are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses. IFNs belong to the large class of proteins known as cytokines, molecules used for communication between cells to trigger the protective defenses of the immune system that help eradicate pathogens. Interferons are named for their ability to "interfere" with viral replication by protecting cells from virus infections. However, virus-encoded genetic elements have the ability to antagonize the IFN response contributing to viral pathogenesis and viral diseases. IFNs also have various other functions: they activate immune cells, such as natural killer cells and macrophages, and they increase host defenses by up-regulating antigen presentation by virtue of increasing the expression of major histocompatibility complex (M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonuclease L
Ribonuclease L or RNase L (for ''latent''), known sometimes as ribonuclease 4 or 2'-5' oligoadenylate synthetase-dependent ribonuclease — is an Interferon type I, interferon (IFN)-induced ribonuclease which, upon activation, destroys all RNA within the Cell (biology), cell (both cellular and viral). RNase L is an enzyme that in humans is encoded by the ''RNASEL'' gene. This gene encodes a component of the interferon-regulated 2'-5'oligoadenylate (2'-5'A) system that functions in the antiviral and antiproliferative roles of interferons. RNase L is activated by dimerization, which occurs upon 2'-5'A binding, and results in cleavage of all RNA in the cell. This can lead to activation of MDA5, an RNA helicase involved in the production of interferons. Synthesis and activation RNase L is present in very minute quantities during the normal cell cycle. When interferon binds to cell receptors, it activates transcription of around 300 genes to bring about the antiviral state. Among ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |