|
1836 In Science
The year 1836 in science and technology involved some significant events, listed below. Astronomy * May 15 – Francis Baily, during an eclipse of the Sun, observes the phenomenon named after him as Baily's beads. Biology * October 2 – Naturalist Charles Darwin returns to Falmouth, Cornwall, Falmouth, England, aboard after a 5-year journey collecting biological data he will later use to develop his theory of evolution. * Writer Georg Büchner's dissertation on the common barbel (fish), ''Barbus barbus'', "Mémoire sur le Système Nerveux du Barbeaux (''Cyprinus barbus L.'')" is published in Paris and Strasbourg. In October, after receiving his doctorate, he is appointed by the University of Zurich as a lecturer in anatomy. * Theodor Schwann discovers pepsin in extracts from the stomach lining, the first isolation of an animal enzyme. Chemistry * French chemist Auguste Laurent discovers o-phthalic acid (1,2-benzenecarboxylic acid) by oxidizing naphthalene tetrachloride. * Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Match
A match is a tool for starting a fire. Typically, matches are made of small wooden sticks or stiff paper. One end is coated with a material that can be ignited by friction generated by striking the match against a suitable surface. Wooden matches are packaged in matchboxes, and paper matches are partially cut into rows and stapled into matchbooks. The coated end of a match, known as the match "head", consists of a bead of active ingredients and binder (material), binder, often colored for easier inspection. There are two main types of matches: safety matches, which can be struck only against a specially prepared surface, and strike-anywhere matches, for which any suitably frictional surface can be used. Because of the substance used to coat each match, this makes them non-biodegradable. Etymology Historically, the term ''match'' referred to lengths of rope, cord (later cambric) impregnated with chemicals, and allowed to burn continuously. These were used to light fires and fir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
János Irinyi
János Irinyi (sometimes also spelled ''János Irínyi''; ; 18 May 1817 – 17 December 1895) was a Hungarian chemist and inventor of the noiseless and non-explosive match. He achieved this by mixing the yellow (also called white) phosphorus with lead dioxide instead of the potassium chlorate used previously. this site's mention of calcium chlorate rather than potassium chlorate appears to be an error? Irinyi also took part in the . Asteroid Asteroid 106869 Irinyi, discovered by Hungarian astronome ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hungarian People
Hungarians, also known as Magyars ( ; hu, magyarok ), are a nation and ethnic group native to Hungary () and Kingdom of Hungary, historical Hungarian lands who share a common Hungarian culture, culture, Hungarian history, history, Magyar tribes, ancestry, and Hungarian language, language. The Hungarian language belongs to the Uralic languages, Uralic language family. There are an estimated 15 million ethnic Hungarians and their descendants worldwide, of whom 9.6 million live in today's Hungary. About 2–3 million Hungarians live in areas that were part of the Kingdom of Hungary before the Treaty of Trianon in 1920 and are now parts of Hungary's seven neighbouring countries, Hungarians in Slovakia, Slovakia, Hungarians in Ukraine, Ukraine, Hungarians in Romania, Romania, Hungarians in Serbia, Serbia, Hungarians of Croatia, Croatia, Prekmurje, Slovenia, and Hungarians in Austria, Austria. Hungarian diaspora, Significant groups of people with Hungarian ancestry live in various oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III-V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in rats, ham ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
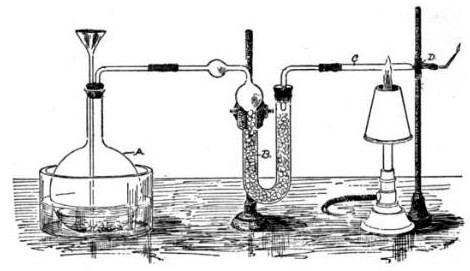
Marsh Test
The Marsh test is a highly sensitive method in the detection of arsenic, especially useful in the field of forensic toxicology when arsenic was used as a poison. It was developed by the chemist James Marsh and first published in 1836. The method continued to be used, with improvements, in forensic toxicology until the 1970s. Arsenic, in the form of white arsenic trioxide , was a highly favored poison, being odourless, easily incorporated into food and drink, and before the advent of the Marsh test, untraceable in the body. In France, it came to be known as ' ("inheritance powder"). For the untrained, arsenic poisoning will have symptoms similar to cholera. Precursor methods The first breakthrough in the detection of arsenic poisoning was in 1775 when Carl Wilhelm Scheele discovered a way to change arsenic trioxide to garlic-smelling arsine gas (AsH3), by treating it with nitric acid (HNO3) and combining it with zinc. :As2O3 + 6 Zn + 12 HNO3 → 2 AsH3 + 6 Zn(NO3)2 + 3 H2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Marsh (chemist)
James Marsh (2 September 1794 – 21 June 1846) was a British chemist who invented the Marsh test for detecting arsenic. Born in Kent, he was working as a labourer in Woolwich in the late 1810s and early 1820s, before joining the Royal Artillery. He was married to Mary, and had four children, two of whom died in infancy. His surviving daughters were Lavinia Bithiah (1821-1896) and Lucretia Victoria (1829-1910). Scientific work While Marsh was most famous for inventing the test that bears his name, he was also a skilled and inventive scientist who held the post of Ordnance Chemist at the Royal Arsenal at Woolwich. He developed the screw time fuze for mortar shells and in 1830 the percussion tube. In 1832 ''HMS Castor'' was the first ship to have her guns modified with these innovations. They were not approved for the Army until 1845, when Woolwich began their manufacture—for coastal artillery only. They became obsolete in 1866. Marsh also worked as an assistant to Michael ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edmund Davy
Edmund Davy FRS (1785 – 5 November 1857)Christopher F. Lindsey, 'Davy, Edmund (1785–1857)’, Oxford Dictionary of National Biography, Oxford University Press, 200 accessed 6 April 2008/ref> was a professor of chemistry at the Royal Cork Institution from 1813 and at the Royal Dublin Society from 1826.Leslie Stephen (Ed.). ''Dictionary of National Biography'', Smith, Elder & Co., London, 1888, Vol. XIV, p.185. He discovered acetylene, as it was later namedAmerican Council of Learned Societies. ''Dictionary of Scientific Biography'', Charles Scribner's Sons, New York, 1981, Vol. 2, p.67. by Marcellin Berthelot. He was also an original member of the Chemical Society, and a member of the Royal Irish Academy. Family and early life Edmund Davy was a cousin of Humphry Davy, the famous chemist who invented the Davy lamp for the safety of miners. Edmund, the son of William Davy, was born in Penzance, Cornwall, and lived there throughout his teen years. He moved to London in 1804 to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he produced a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlenmeye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |