|
11β-Hydroxysteroid Dehydrogenase Type 1
11β-Hydroxysteroid dehydrogenase type 1, also known as cortisone reductase, is an NADPH-dependent enzyme highly expressed in key metabolic tissues including liver, adipose tissue, and the central nervous system. In these tissues, HSD11B1 reduces cortisone to the active hormone cortisol that activates glucocorticoid receptors. It belongs to the family of short-chain dehydrogenases. It is encoded by the gene. Function The protein encoded by this gene is a microsomal enzyme that catalyzes the conversion of the stress hormone cortisol to the inactive metabolite cortisone. In addition, the encoded protein can catalyze the reverse reaction, the conversion of cortisone to cortisol. Too much cortisol can lead to central obesity, and a particular variation in this gene has been associated with obesity and insulin resistance in children. Two transcript variants encoding the same protein have been found for this gene. Clinical significance 11β-HSD1 is inhibited by carbenoxolone, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life. NADPH is the reduced form of NADP. NADP differs from NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, and NADP+ phosphatase can convert NADPH back to NADH to maintain a balance. Some forms of the NAD+ kinas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corticosteroid 11-beta-dehydrogenase Isozyme 2
Corticosteroid 11-β-dehydrogenase isozyme 2 also known as 11-β-hydroxysteroid dehydrogenase 2 is an enzyme that in humans is encoded by the gene. Function Corticosteroid 11-β-dehydrogenase isozyme 2 is an NAD+-dependent enzyme expressed in aldosterone-selective epithelial tissues such as the kidney, colon, salivary and sweat glands. HSD211B2 expression is also found in the brainstem in a small, aldosterone-sensitive subset of neurons located in the nucleus of the solitary tract referred to as HSD2 neurons. In these tissues, HSD11B2 oxidizes the glucocorticoid cortisol to the inactive metabolite cortisone, thus preventing illicit activation of the mineralocorticoid receptor. This protective mechanism is necessary because cortisol circulates at 100- to 1000-fold higher concentrations than aldosterone, and binds with equal affinity to the mineralocorticoid receptor, thereby out-competing aldosterone in cells that do not produce HSD11B2. This glucocorticoid-inactivating enzyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cortisone Reductase Deficiency
Cortisone reductase deficiency is caused by dysregulation of the 11β-hydroxysteroid dehydrogenase type 1 enzyme (11β-HSD1), otherwise known as cortisone reductase, a bi-directional enzyme, which catalyzes the interconversion of cortisone to cortisol in the presence of NADH as a co-factor. If levels of NADH are low, the enzyme catalyses the reverse reaction, from cortisol to cortisone, using NAD+ as a co-factor. Cortisol is a glucocorticoid that plays a variety of roles in many different biochemical pathways, including, but not limited to: gluconeogenesis, suppressing immune system responses and carbohydrate metabolism. One of the symptoms of cortisone reductase deficiency is hyperandrogenism, resulting from activation of the Hypothalamic–pituitary–adrenal axis. The deficiency has been known to exhibit symptoms of other disorders such as Polycystic Ovary Syndrome in women. Cortisone Reductase Deficiency alone has been reported in fewer than ten cases in total, all but one ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HDAC Inhibitors
Histone deacetylase inhibitors (HDAC inhibitors, HDACi, HDIs) are chemical compounds that inhibit histone deacetylases. HDIs have a long history of use in psychiatry and neurology as mood stabilizers and anti-epileptics. More recently they are being investigated as possible treatments for cancers, parasitic and inflammatory diseases. Cellular biochemistry/pharmacology To carry out gene expression, a cell must control the coiling and uncoiling of DNA around histones. This is accomplished with the assistance of histone acetyl transferases (HAT), which acetylate the lysine residues in core histones leading to a less compact and more transcriptionally active euchromatin, and, on the converse, the actions of histone deacetylases (HDAC), which remove the acetyl groups from the lysine residues leading to the formation of a condensed and transcriptionally silenced chromatin. Reversible modification of the terminal tails of core histones constitutes the major epigenetic mechanism for re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Tea
Green tea is a type of tea that is made from '' Camellia sinensis'' leaves and buds that have not undergone the same withering and oxidation process which is used to make oolong teas and black teas. Green tea originated in China, and since then its production and manufacture has spread to other countries in East Asia. Several varieties of green tea exist, which differ substantially based on the variety of ''C. sinensis'' used, growing conditions, horticultural methods, production processing, and time of harvest. The two main components unique to green tea are "catechins" and "theanine," and the health effects of these components are attracting a great deal of attention in Japan and abroad. History Tea consumption has its legendary origins in China during the reign of mythological Emperor Shennong. A book written by Lu Yu in 618–907 AD (Tang dynasty), ''The Classic of Tea'' (), is considered important in green tea history. The ''Kissa Yōjōki'' (喫茶養生記 ''Book ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epigallocatechin Gallate
Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin. EGCG – the most abundant catechin in tea – is a polyphenol under basic research for its potential to affect human health and disease. EGCG is used in many dietary supplements. Food sources Tea It is found in high content in the dried leaves of green tea (7380 mg per 100 g), white tea (4245 mg per 100 g), and in smaller quantities, black tea (936 mg per 100 g). During black tea production, the catechins are mostly converted to theaflavins and thearubigins via polyphenol oxidases. Other Trace amounts are found in apple skin, plums, onions, hazelnuts, pecans, and carob powder (at 109 mg per 100 g). Bioavailability When taken orally, EGCG has poor absorption even at daily intake equivalent to 8–16 cups of green tea, an amount causing adverse effects such as nausea or heartburn. After consumption, EGCG ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salicylate
Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substances Control Act (TSCA) Chemical Substance Inventory as an experimental teratogen. The name is from Latin ''salix'' for willow tree. It is an ingredient in some anti-acne products. Salts and esters of salicylic acid are known as salicylates. Uses Medicine Salicylic acid as a medication is commonly used to remove the outer layer of the skin. As such, it is used to treat warts, psoriasis, acne vulgaris, ringworm, dandruff, and ichthyosis. Similar to other hydroxy acids, salicylic acid is an ingredient in many skincare products for the treatment of seborrhoeic dermatitis, acne, psoriasis, calluses, corns, keratosis pilaris, acanthosis nigricans, ichthyosis, and warts. Uses in manufacturing Salicylic acid is used as a food preservativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycyrrhiza Glabra
Liquorice (British English) or licorice (American English) ( ; also ) is the common name of ''Glycyrrhiza glabra'', a flowering plant of the bean family Fabaceae, from the root of which a sweet, aromatic flavouring can be extracted. The liquorice plant is an herbaceous perennial legume native to Western Asia, North Africa, and Southern Europe. Botanically, it is not closely related to anise or fennel, which are sources of similar flavouring compounds. (Another such source, star anise, is even more distantly related from anise and fennel than liquorice, despite its similar common name.) Liquorice is used as a flavouring in candies and tobacco, particularly in some European and West Asian countries. Liquorice extracts have been used in herbalism and traditional medicine. Excessive consumption of liquorice (more than per day of pure glycyrrhizinic acid, a liquorice component) may result in adverse effects, and overconsumption should be suspected clinically in patients presentin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycyrrhizic Acid
Glycyrrhizin (or glycyrrhizic acid or glycyrrhizinic acid) is the chief sweet-tasting constituent of ''Glycyrrhiza glabra'' (liquorice) root. Structurally, it is a saponin used as an emulsifier and gel-forming agent in foodstuffs and cosmetics. Its aglycone is enoxolone. Pharmacokinetics After oral ingestion, glycyrrhizin is hydrolysed to 18β-glycyrrhetinic acid (enoxolone) by intestinal bacteria. After absorption from the gut, 18β-glycyrrhetinic acid is metabolised to 3β-monoglucuronyl-18β-glycyrrhetinic acid in the liver. This metabolite circulates in the bloodstream. Consequently, its oral bioavailability is poor. Most of it is eliminated by bile and only a minor part (0.31–0.67%) by urine. After oral ingestion of 600 mg of glycyrrhizin the metabolite appeared in urine after 1.5 to 14 hours. Maximal concentrations (0.49 to 2.69 mg/L) were achieved after 1.5 to 39 hours and metabolite can be detected in the urine after 2 to 4 days. Flavouring properties Glycyrrhizin i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
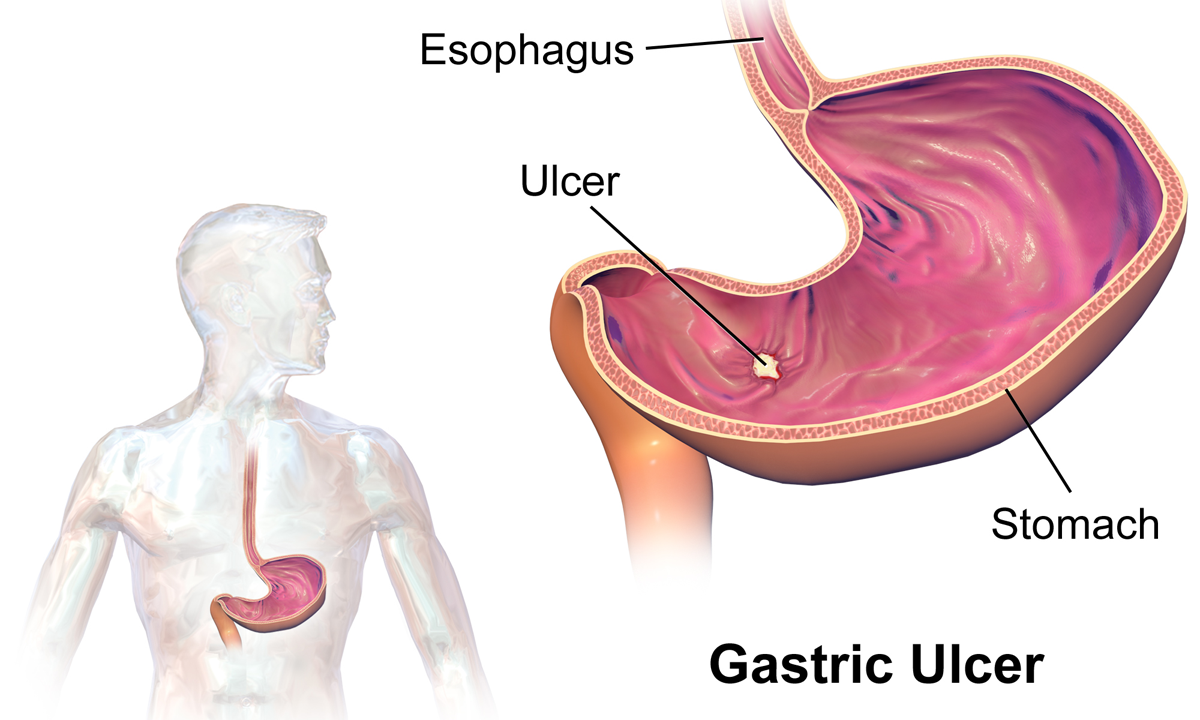
Peptic Ulcer
Peptic ulcer disease (PUD) is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines is a duodenal ulcer. The most common symptoms of a duodenal ulcer are waking at night with upper abdominal pain and upper abdominal pain that improves with eating. With a gastric ulcer, the pain may worsen with eating. The pain is often described as a burning or dull ache. Other symptoms include belching, vomiting, weight loss, or poor appetite. About a third of older people have no symptoms. Complications may include bleeding, perforation, and blockage of the stomach. Bleeding occurs in as many as 15% of cases. Common causes include the bacteria ''Helicobacter pylori'' and non-steroidal anti-inflammatory drugs (NSAIDs). Other, less common causes include tobacco smoking, stress as a result of other serious health conditions, Behçet's di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenoxolone
Carbenoxolone (CBX) is a glycyrrhetinic acid derivative with a steroid-like structure, similar to substances found in the root of the licorice plant. Carbenoxolone is used for the treatment of peptic, esophageal and oral ulceration and inflammation. Electrolyte imbalance is a serious side effect of carbenoxolone when used systemically. Carbenoxolone reversibly inhibits the conversion of inactive cortisone to cortisol by blocking 11β-hydroxysteroid dehydrogenase (11β-HSD). 11β-HSD also reversibly catalyzes the conversion of 7-ketocholesterol to 7-beta-hydroxycholesterol. Carbenoxolone is a modestly potent, reasonably effective, water-soluble blocker of gap junctions. Carbenoxolone has also been used in topical creams such as Carbosan gel, marketed for treatment of lip sores and mouth ulcers. Nootropic effects Carbenoxolone has also been investigated for nootropic effects. This research started from an observation that long-term exposure to glucocorticoids may have negative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)