|
1,2,7-Trihydroxyanthraquinone
Anthrapurpurin, or 1,2,7-trihydroxyanthraquinone, is a purple dye used in histology for the detection of calcium. See also * * Purpurin * * * |
Trihydroxyanthraquinone
A trihydroxyanthraquinone or trihydroxyanthracenedione is any of several isomeric organic compounds with formula , formally derived from anthraquinone by replacing three hydrogen atoms by hydroxyl groups. They include several historically important dyes. Wahl, Andre; Atack, F. W (1919) ''The Manufacture Of Organic Dyestuffs''. G. Bell And Sons, LimitedOnline versionaccessed on 2010-01-22. Hugh Alister McGuigan (1921), ''An introduction to chemical pharmacology; pharmacodynamics in relation to chemistry''. P. Blakiston's son, PhiladelphiaOnline versionat archive.org, accessed on 2010-01-30. The isomers may differ in the parent anthraquinone isomer and/or of the three hydroxyl groups. In general there are 56 ways of choosing three out of the 8 hydrogens. However, if the underlying core is symmetrical, some of these choices will give identical molecules. Isomers From 9,10-anthraquinone Due to the symmetry of the 9,10-anthraquinone core, there are only 14 isomers. CRC (1996) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
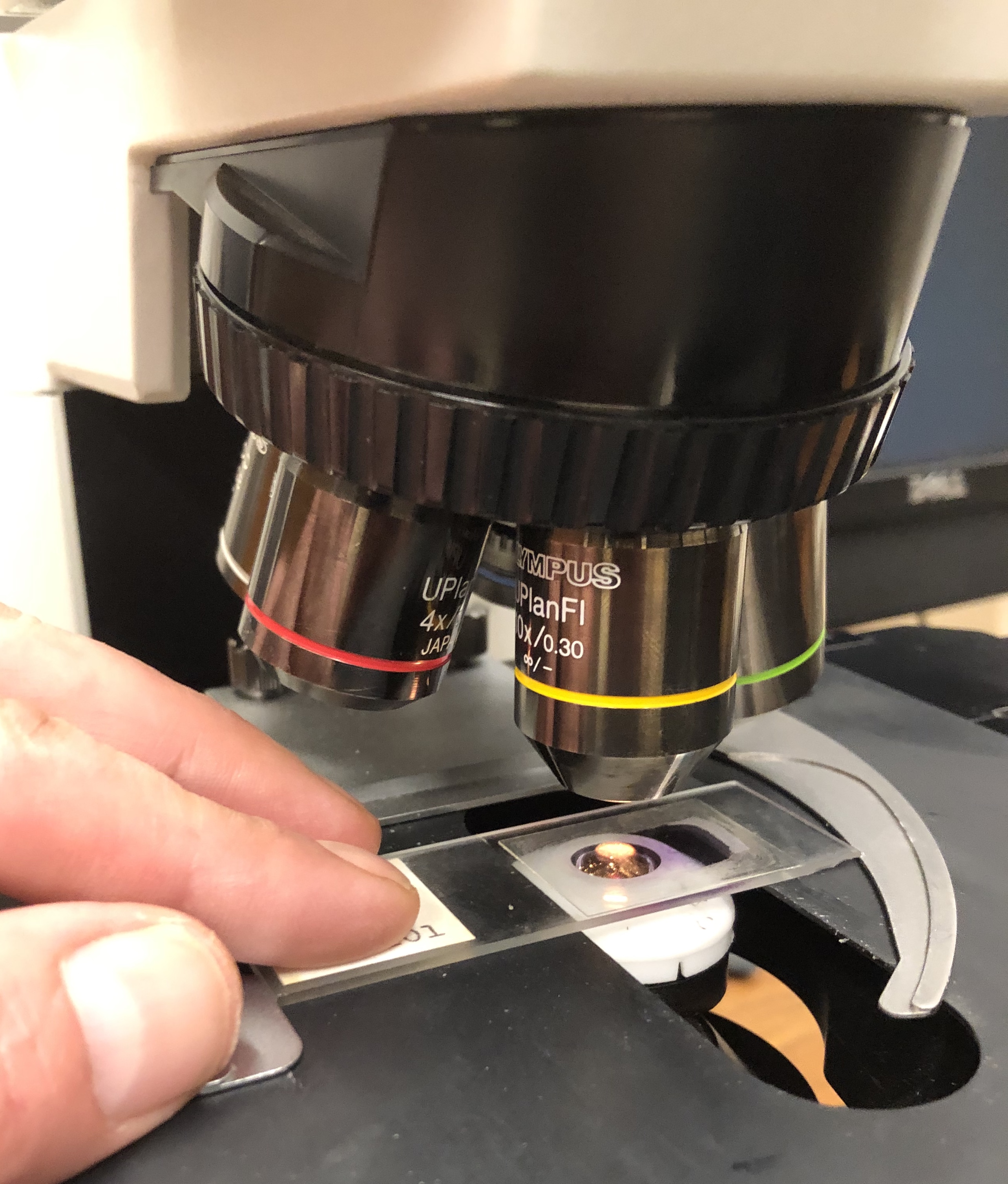
Histology
Histology, also known as microscopic anatomy or microanatomy, is the branch of biology which studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into ''organology'', the study of organs, ''histology'', the study of tissues, and ''cytology'', the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms. Biological tissues Animal tissue classification There are four basic types of animal tissues: muscle tissue, nervous tissue, connective tissue, and epithelial tissue. All animal tissues are considered to be subtypes of these four principal tissue types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alizarin
Alizarin (also known as 1,2-dihydroxyanthraquinone, Mordant Red 11, C.I. 58000, and Turkey Red) is an organic compound with formula that has been used throughout history as a prominent red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus.The primary madder species from which alizarin historically has been obtained is ''Rubia tinctorum''. See also In 1869, it became the first natural dye to be produced synthetically. Alizarin is the main ingredient for the manufacture of the madder lake pigments known to painters as rose madder and alizarin crimson. Alizarin in the most common usage of the term has a deep red color, but the term is also part of the name for several related non-red dyes, such as Alizarine Cyanine Green and Alizarine Brilliant Blue. A notable use of alizarin in modern times is as a staining agent in biological research because it stains free calcium and certain calcium compounds a red or ligh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purpurin (dye)
1,2,4-Trihydroxyanthraquinone, commonly called purpurin, is an natural anthraquinone, anthraquinone. It is a natural dye, naturally occurring red/yellow dye. It is formally derived from 9,10-anthraquinone by replacement of three hydrogen atoms by hydroxyl (OH) groups. Purpurin is also called verantin, smoke Brown G, hydroxylizaric acid, and C.I. 58205. It is a minor component of the classical lake pigment "madder lake" or Rose madder, Rose Madder. History Madder root has been used for dying cloth at least since 1500 BC.Madder Root'' catalog entry at Natural Pigments website. Accessed on 2010-01-22. Purpurin and alizarin were isolated from the root by Pierre Robiquet and Jean Jacques Colin, Colin, two French chemists, in 1826. They were identified as anthracene derivatives by Carl Gräbe, Gräbe and Carl Theodore Liebermann, Liebermann in 1868. They also synthesized alizarin from bromoanthraquinone, which, together with the conversion of alizarin into purpurin published ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyanthraquinone
A hydroxyanthraquinone (formula: C14H9O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). The IUPAC nomenclature recommends hydroxyanthracenedione. Usually "hydroxyanthraquinone" refers to a derivative of 9,10-anthraquinone. Quoted by Khalafy and Bruce. Quoted by Khalafy and Bruce. Isomers In general, the term may mean any anthraquinone derivative where any number ''n'' of hydrogens have been replaced by ''n'' hydroxyls, so that the formula is . In this case, the number ''n'' (which is between 1 and 8) is indicated by a multiplier prefix A prefix is an affix which is placed before the Word stem, stem of a word. Adding it to the beginning of one word changes it into another word. For example, when the prefix ''un-'' is added to the word ''happy'', it creates the word ''unhappy'' ... (mono-, di-, tri-, up to octa-). Additional hydroxy- compounds can be derived f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula . Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone (IUPAC: 9,10-dioxoanthracene) wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite. Synthesis There are several current industrial methods to produce 9,10-anthraquinone: # The oxidation of anthracene. Chromium(VI) is the typical oxidant. # The Friedel-Crafts reaction of benzene and phthalic anhydride in presence of AlCl3. o-Benzoylbenzoic acid is an intermediate. This reaction is useful for produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |