|
1,1'-Bis(diphenylphosphino)ferrocene
1,1-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe). Preparation This compound is commercially available. It may be prepared by treating dilithioferrocene with chlorodiphenylphosphine: :Fe(CHLi) + 2 ClPPh → Fe(CHPPh) + 2 LiCl The dilithiation of ferrocene is easily achieved with ''n''-butyllithium in the presence of TMEDA. Many related ligands can be made in this way. The Fe center is typically not involved in the behavior of the ligand. Reactions Dppf readily forms metal complexes. The palladium derivative, (dppf)PdCl, which is popular for palladium-catalyzed coupling reactions, is prepared by treating dppf with the acetonitrile or benzonitrile adducts of palladium dichloride: Substitution of the phenyl substituents in dppf leads to deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
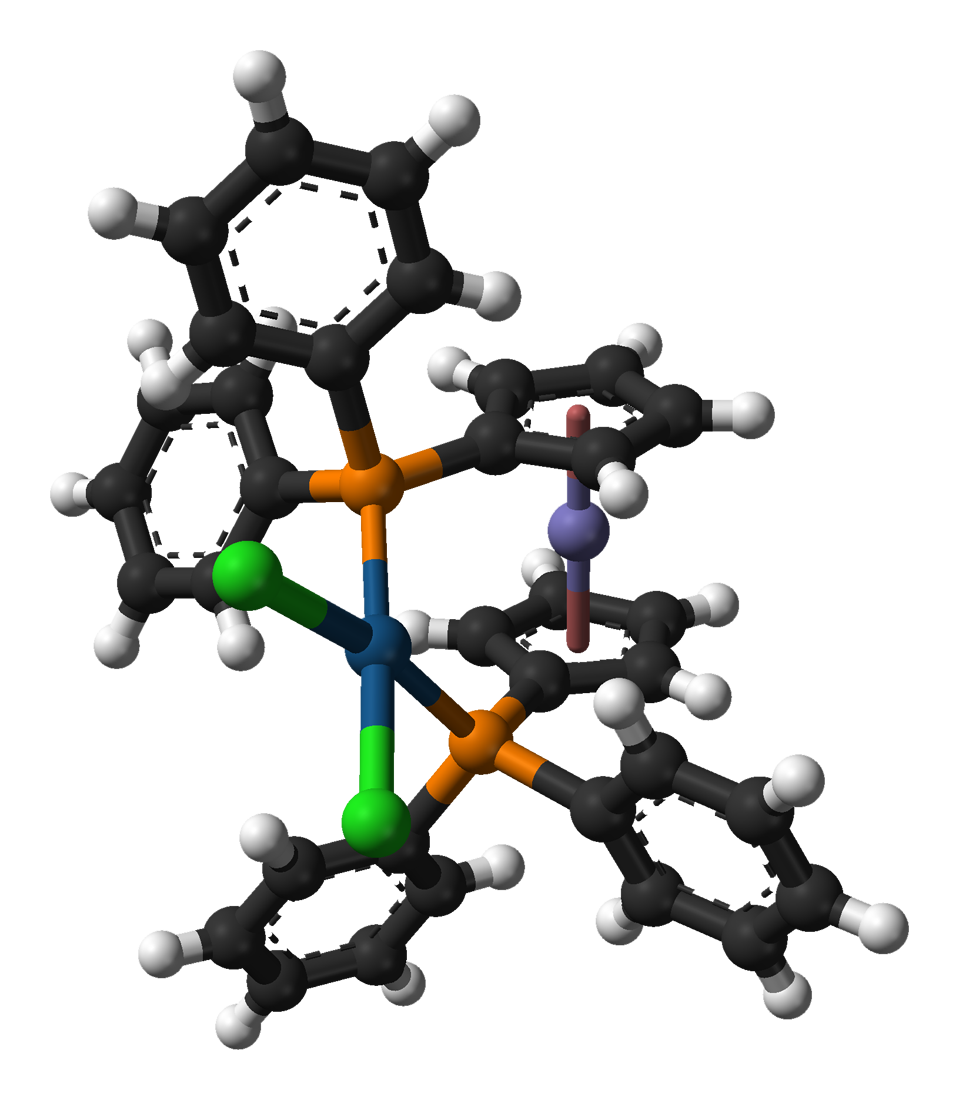
(1,1'-Bis(diphenylphosphino)ferrocene)palladium(II) Dichloride
,1'‑Bis(diphenylphosphino)ferrocenealladium(II) dichloride is a palladium complex containing the bidentate ligand 1,1'-bis(diphenylphosphino)ferrocene (dppf), abbreviated as dppf)PdCl This commercially available material can be prepared by reacting dppf with a suitable nitrile complex of palladium dichloride: :dppf + PdCl(RCN) → (dppf)PdCl + 2 RCN (RCN = CHCN or CHCN) The compound is popularly used for palladium-catalyzed coupling reactions, such as the Buchwald–Hartwig amination and the reductive homocoupling of aryl halide In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exh ...s. References {{DEFAULTSORT:Bis(diphenylphosphino)ferrocene)palladium(II) dichloride, (1,1'- Palladium compounds Phosphine complexes Catalysts Ferrocenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
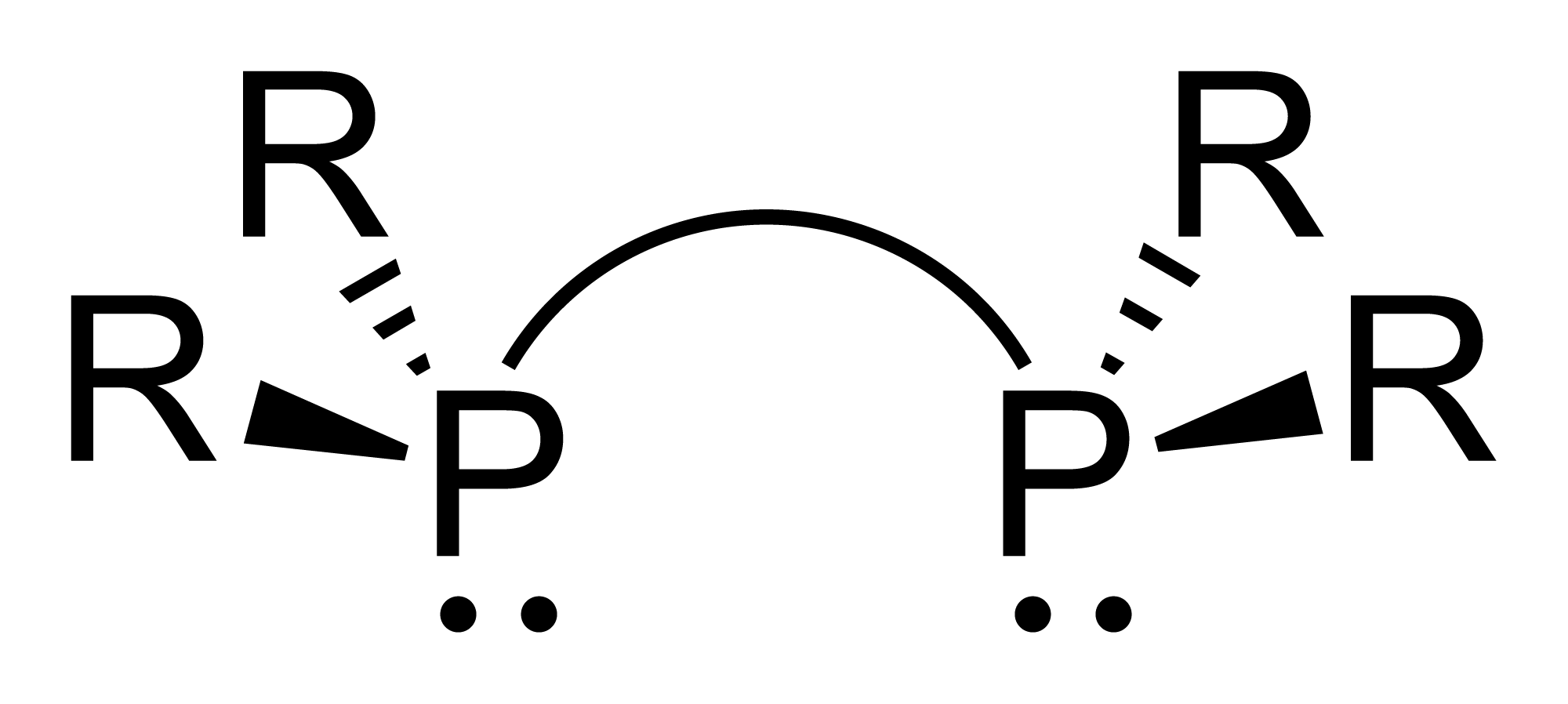
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dilithioferrocene
1,1'-Dilithioferrocene is the organoiron compound with the formula Fe(C5H4Li)2. It is exclusively generated and isolated as a solvate, using either ether or tertiary amine ligands bound to the lithium centers. Regardless of the solvate, dilithioferrocene is used commonly to prepare derivatives of ferrocene. Synthesis and reactions Treatment of ferrocene with butyl lithium gives 1,1'-dilithioferrocene, regardless of the stoichiometry (monolithioferrocene requires special conditions for its preparation). Typically the lithiation reaction is conducted in the presence of tetramethylethylenediamine (tmeda). The adduct e(C5H4Li)2sub>3(tmeda)2 has been crystallized from such solutions. Recrystallization of this adduct from thf gives e(C5H4Li)2sub>3(thf)6. 1,1'-Dilithioferrocene reacts with a variety of electrophiles to afford disubstituted derivatives of ferrocene. These electrophiles include S8 (to give 1,1'-ferrocenetrisulfide), chlorophosphines, and chlorosilanes. The di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium Dichloride
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures. Structure Two forms of PdCl2 are known, denoted α and β. In both forms, the palladium centres adopt a square-planar coordination geometry that is characteristic of Pd(II). Furthermore, in both forms, the Pd(II) centers are linked by μ2-chloride bridges. The α-form of PdCl2 is a polymer, consisting of "infinite" slabs or chains. The β-form of PdCl2 is molecular, consisting of an octahedral cluster of six Pd atoms. Each of the twelve edges of this octahedron is spanned by Cl−. PtCl2 adopts similar structures, whereas NiCl2 adopts the CdCl2 motif, featuring hexacoordinated Ni(II). Two further polymorphs, γ-PdCl2 a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borrowing Hydrogen
Hydrogen auto-transfer, also known as borrowing hydrogen, is the activation of a chemical reaction by temporary transfer of two hydrogen atoms from the reactant to a catalyst and return of those hydrogen atoms back to a reaction intermediate to form the final product. Two major classes of borrowing hydrogen reactions exist: (a) those that result in hydroxyl substitution, and (b) those that result in carbonyl addition. In the former case, alcohol dehydrogenation generates a transient carbonyl compound that is subject to condensation followed by the return of hydrogen. In the latter case, alcohol dehydrogenation is followed by reductive generation of a nucleophile, which triggers carbonyl addition. As borrowing hydrogen processes avoid manipulations otherwise required for discrete alcohol oxidation and the use of stoichiometric organometallic reagents, they typically display high levels of atom-economy and, hence, are viewed as examples of Green chemistry. History The Guerbet react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buchwald Synthesis
Buchwald is a German and Jewish surname. Notable people with the surname include: *Art Buchwald (1925–2007), American humorist * Charles Buchwald (1880–1951), Danish amateur footballer * Dave Buchwald (born 1970), Computer hacker, filmmaker, artist * David Buchwald (born 1970), American politician * Dedra Buchwald, American epidemiologist, doctor, and professor *Ephraim Buchwald, American Orthodox rabbi *Gerhard Buchwald (1920–2009), German medical doctor and vaccination critic *Guido Buchwald (born 1961), German footballer and manager * Harold Buchwald (1928–2008), Canadian lawyer * Jed Buchwald, American historian *Manuel Buchwald (born 1940), Peruvian-Canadian geneticist and academic *Naomi Reice Buchwald (born 1944), American jurist, federal judge * Nathaniel Buchwald (1890–1956), American cultural critic and translator * Nathaniel A. Buchwald (1924–2006), American neuroscientist, educator and administrator * Péter Bakonyi (Buchwald) (born 1938), Hungarian Olympic fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzonitrile
Benzonitrile is the chemical compound with the formula , abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine. Production It is prepared by ammoxidation of toluene, that is its reaction with ammonia and oxygen (or air) at . : + 3/2 + → + In the laboratory it can be prepared by the dehydration of benzamide or by the Rosenmund–von Braun reaction using cuprous cyanide or NaCN/DMSO and bromobenzene. : Applications Laboratory uses Benzonitrile is a useful solvent and a versatile precursor to many derivatives. It reacts with amines to afford N-substituted benzamides after hydrolysis. It is a precursor to diphenylketimine (b.p. 151 °C, 8 mm Hg) via reaction with phenylmagnesium bromide followed by methanolysis. Benzonitrile forms coordination complexes with transition metals that are both soluble in organic solvents and conveniently labile. One example is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-chloride-xtal-3D-balls.png)