|
1,4-didehydrobenzene
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydroarenes in this case), although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes. Bonding in arynes The alkyne representation of benzyne is the most widely encountered. Arynes are usually described as having a strained triple bond. Geometric constraints on the triple bond in benzyne result in diminished overlap of in-plane p-orbitals, and thus weaker triple bond. The vibrational frequency of the triple bond in benzyne was assigned by Radziszewski to be 1846 cm−1, indicating a weaker triple bond than in unstrained alkyne with vibrational frequency of approximately 2150 cm−1. Nevertheless, benzyne is more like a strained alkyne than a diradical, as seen from the large singlet–triplet gap and alkyne-like reactivity. The HOMO and LUMO, LUMO of aryne lies much lower than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ring
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many non- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ''n''-butyl triflate can be written as . The corresponding triflate anion, , is an extremely stable polyatomic ion; this comes from the fact that triflic acid () is a superacid; i.e. it is more acidic than pure sulfuric acid, already one of the strongest acids known. Applications A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution, Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in SN2 reactions, they must be stored in conditions free of nucleophiles (such as water). The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regiochemistry2
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where on a substituted benzene ring a further substituent will be added. A specific example is a halohydrin formation reaction with 2-propenylbenzene: : Because of the preference for the formation of one product over another, the reaction is selective. This reaction is regioselective because it selectively generates one constitutional isomer rather than the other. Various examples of regioselectivity have been formulated as rules for certain classes of compounds under certain conditions, many of which are named. Among the first introduced to chemistry students are Markovnikov's rule for the addition of protic acids to alkenes, and the Fürst-Plattner rule for the addition of nucleophiles to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond Generation1
Triple is used in several contexts to mean "threefold" or a "treble": Sports * Triple (baseball), a three-base hit * A basketball three-point field goal * A figure skating jump with three rotations * In bowling terms, three strikes in a row * In cycling, a crankset with three chainrings Places * Triple Islands, an uninhabited island group in Nunavut, Canada * Triple Island, British Columbia, Canada * Triple Falls (other), four waterfalls in the United States & Canada * Triple Glaciers, in Grand Teton National Park, Wyoming * Triple Crossing, Richmond, Virginia, believed to be the only place in North America where three Class I railroads cross * Triple Bridge, a stone arch bridge in Ljubljana, Slovenia Transportation * Kawasaki triple, a Japanese motorcycle produced between 1969 and 1980 * Triumph Triple, a motorcycle engine from Triumph Motorcycles Ltd * A straight-three engine * A semi-truck with three trailers Science and technology * Triple (mathematics) (3-tuple), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Cyanide
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.H. Wayne Richardson "Copper Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Structure Copper cyanide is a coordination polymer. It exists in two polymorphs both of which contain - u-CN chains made from linear copper(I) centres linked by cyanide bridges. In the high-temperature polymorph, HT-CuCN, which is isostructural with AgCN, the linear chains pack on a hexagonal lattice and adjacent chains are off set by +/- 1/3 ''c'', Figure 1. In the low-temperature polymorph, LT-CuCN, the chains deviate from linearity and pack into rippled layers which pack in an AB fashion with chains in adjacent layers rotated by 49 °, Figure 2. File:Structure of HT-C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain-growth Polymerization
Chain-growth polymerization (American English, AE) or chain-growth polymerisation (British English, BE) is a polymerization technique where Unsaturated compound, unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics. Introduction In 1953, Paul Flory first classified polymerization as "step-growth polymerization" and "chain-growth polymerization". IUPAC recommends to further simplify "chain-growth polymerization" to "chain polymerization". It is a kind of polymerization where an active center (free radical or ion) is formed, and a plurality of monomers can be polymerized together in a short period of time to form a macromolecule having a large molecular weight. In addition to the regenerated active sites of each monomer unit, polymer growth will only occur at one (or possibly more) endpoint. Many comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
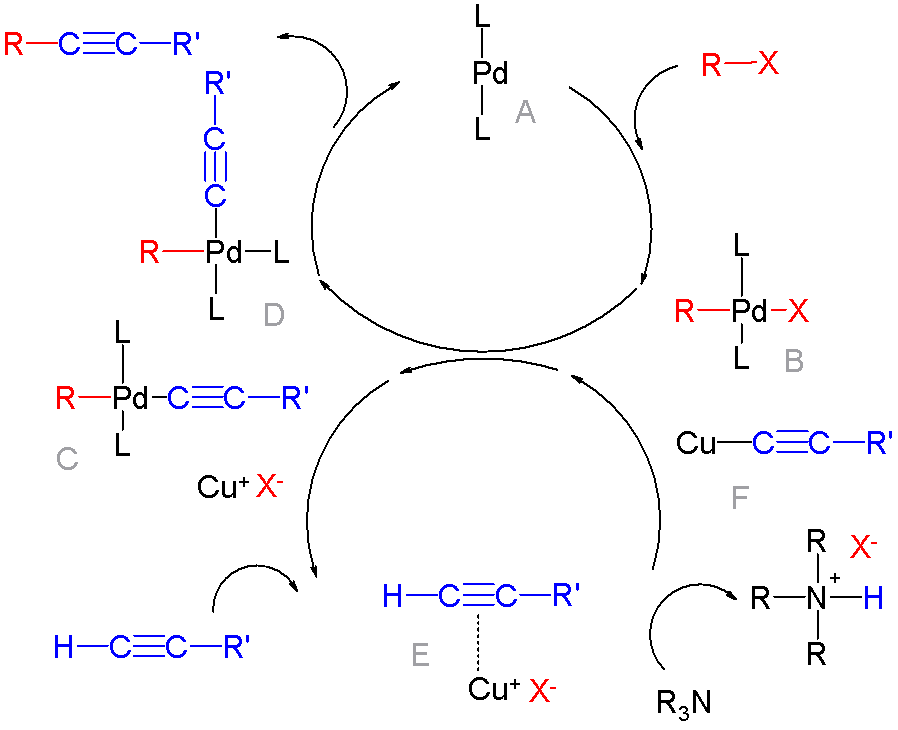
Cross-coupling Of Arynes
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subsequ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuc Addn To Benzyne
Nucs, or nucleus colonies, are small honey bee colonies created from larger colonies, packages, or captured swarms. A nuc hive is centered on a queen bee, the nucleus of the honey bee colony. Layout A nuc hive has all the features of a standard 10 frame Langstroth hive, except for a reduced width. A typical nuc has 5 Langstroth frames arranged side-by-side. Nucs can also be created using other hive dimensions, with the British modified national hive being the most common in the United Kingdom. According to FERA's (Food and Environment Research Agency) National Bee Unit guidelines, the nucleus should be between 3-6 frames of bees, including a queen, workers, brood in all stages, and honey stores.https://secure.fera.defra.gov.uk/beebase/downloadNews.cfm?id=119 {{Bare URL PDF, date=March 2022 Creation The nuc box, also called a nuc, is a smaller version of a normal beehive, designed to hold fewer frames. A smaller space makes it easier for the bees to control the temperature and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead(IV) Acetate
Lead(IV) acetate or lead tetraacetate is an organometallic compound with chemical formula . It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis. Structure In the solid state the lead(IV) centers are coordinated by four acetate ions, which are bidentate, each coordinating via two oxygen atoms. The lead atom is 8 coordinate and the O atoms form a flattened trigonal dodecahedron. Preparation It is typically prepared by treating of red lead with acetic acid and acetic anhydride (), which absorbs water. The net reaction is shown: :Pb3O4 + 4 Ac2O -> Pb(OAc)4 + 2 Pb(OAc)2 The remaining lead(II) acetate can be partially oxidized to the tetraacetate: :2 Pb(OAc)2 + Cl2 -> Pb(OAc)4 + PbCl2 Reagent in organic chemistry Lead tetraacetate is a strong oxidizing agent, a source of acetyloxy groups and a general reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine-O-sulfonic Acid
Hydroxylamine-''O''-sulfonic acid (HOSA) is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum. It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic compound, alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles. Preparation According to a laboratory procedure hydroxylamine-''O''-sulfonic acid can be prepared by treating hydroxylamine sulfate with fuming sulfuric acid (oleum). The industrial process is similar. :(NH3OH)2SO4 + 2SO3 → 2H2NOSO3H + H2SO4 The sulfonation of hydroxylamine can also be effected with chlorosulfonic acid by a method first pub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzotriazole
Benzotriazole (BTA) is a heterocyclic compound with the chemical formula C6H5N3. Its five-membered ring contains three consecutive nitrogen atoms. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and triazole. This white-to-light tan solid has a variety of uses, for instance, as a corrosion inhibitor for copper. Structure Benzotriazole features two fused rings. Its five-membered ring can exist in tautomers A and B, and the derivatives of both tautomers, structures C and D, can also be produced: : Various structural analyses with UV, IR and 1H-NMR spectra indicated that isomer A is predominantly present at room temperature. The bond between positions 1 and 2 and the one between positions 2 and 3 have proved to have the same bond properties. Moreover, the proton does not tightly bind to any of the nitrogen atoms, but rather migrates rapidly between positions 1 and 3. Therefore, the BTA can lose a proton to act as a weak acid (pKa = 8.2) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)