|
öÇ-Decalactone
öÇ-Decalactone (DDL) is a chemical compound, classified as a lactone, that naturally occurs in fruit and milk products in traces. It can be obtained from both chemical and biological sources. Chemically, it is produced from BaeyerãVilliger oxidation of delfone. From biomass, it can be produced via the hydrogenation of 6-amyl-öÝ-pyrone. DDL has applications in food, polymer, and agricultural industries to formulate important products. The S-enantiomer smells good. The R-enantiomer is the main component of the warning stench of the North American porcupine. See also * ö°-Decalactone ''gamma''-Decalactone is a lactone and aroma compound with the chemical formula C10H18O2. It has an intense-peach flavor. It is present naturally in many fruits and fermented products. It is particularly important in the formulation of peach, a ... References {{DEFAULTSORT:Decalactone, öÇ- Delta-lactones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
North American Porcupine
The North American porcupine (''Erethizon dorsatum''), also known as the Canadian porcupine, is a large quill-covered rodent in the New World porcupine family. It is the second largest rodent in North America, after the North American beaver (''Castor canadensis''). The porcupine is a caviomorph rodent whose ancestors crossed the Atlantic from Africa to Brazil 30 million years ago, and then migrated to North America during the Great American Interchange after the Isthmus of Panama rose 3 million years ago. Etymology The word "porcupine" comes from the middle or old French word , which means 'thorn pig'. Its roots derive from the Latin words or pig and meaning thorns. Other colloquial names for the animal include quill pig. It is also referred to as the Canadian porcupine or common porcupine. The porcupine's scientific name, ''Erethizon dorsatum'', can be loosely translated as "the animal with the irritating back". Native American terms for it include the Lakota name meaning q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ö°-Decalactone
''gamma''-Decalactone is a lactone and aroma compound with the chemical formula C10H18O2. It has an intense-peach flavor. It is present naturally in many fruits and fermented products. It is particularly important in the formulation of peach, apricot, and strawberry flavors. It is used as a flavoring for beverages, personal care, pharmaceutical and household goods, as well as a food additive. See also * öÇ-Decalactone öÇ-Decalactone (DDL) is a chemical compound, classified as a lactone, that naturally occurs in fruit and milk products in traces. It can be obtained from both chemical and biological sources. Chemically, it is produced from BaeyerãVilliger oxid ... References Gamma-lactones {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered. Lactones with three- or four-membered rings (öÝ-lactones and öý-lactones) are very reactive, making their isolation difficult. Special methods are normally required for the laboratory synthesis of small-ring lactones as well as those that contain rings larger than six-membered. Nomenclature Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle ã that is, the distance between the relevant -OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
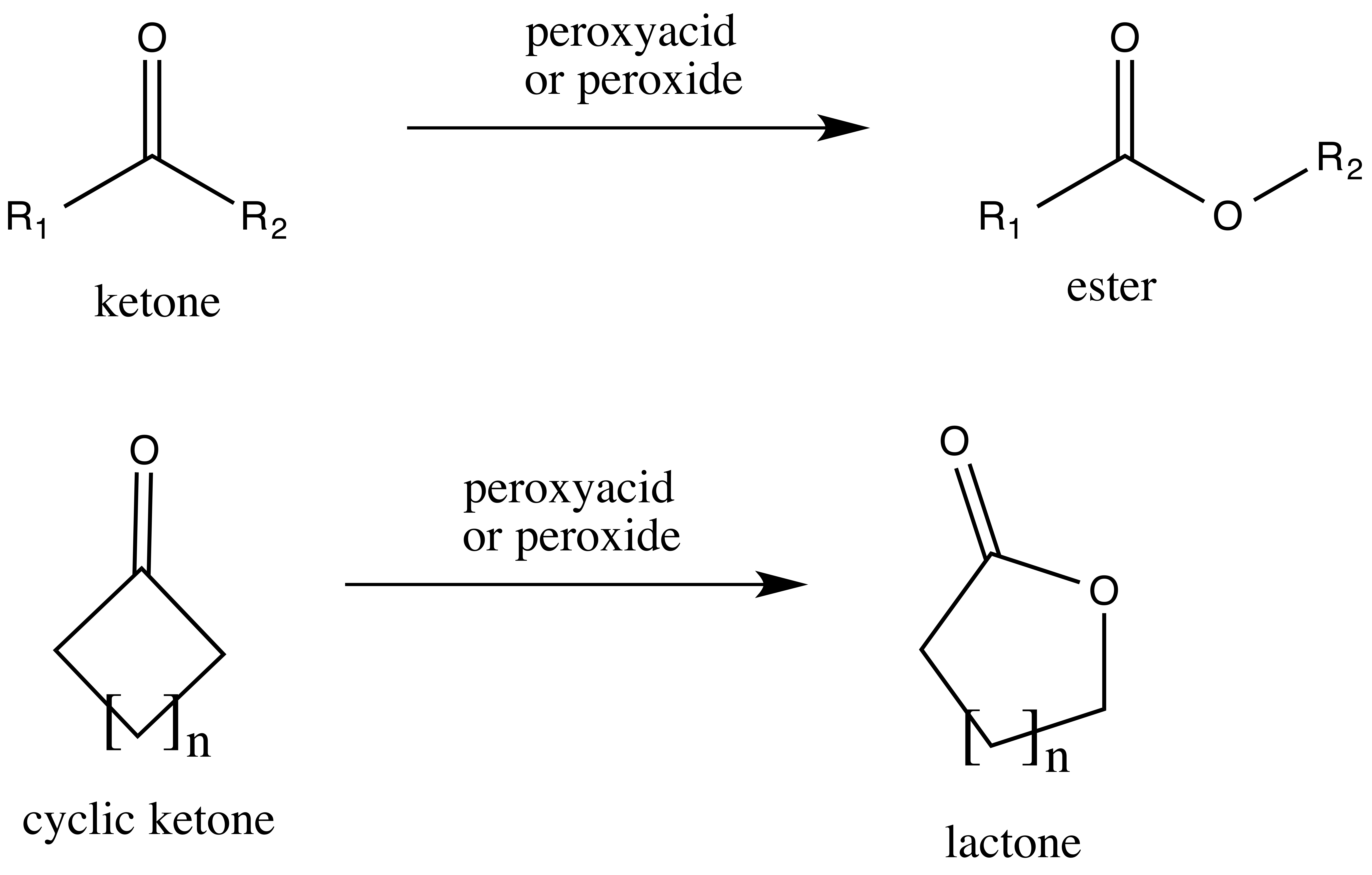
BaeyerãVilliger Oxidation
The BaeyerãVilliger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899. Reaction mechanism In the first step of the reaction mechanism, the peroxyacid protonates the oxygen of the carbonyl group. This makes the carbonyl group more susceptible to be attacked by the peroxyacid. Next, the peroxyacid attacks the carbon of the carbonyl group forming what is known as the Criegee intermediate. Through a concerted mechanism, one of the substituents on the ketone group migrates to the oxygen of the peroxide group while a carboxylic acid leaves. This migration step is thought to be the rate determining step. Finally, deprotonation of the oxocarbenium ion produces the ester. The products of the BaeyerãVilliger oxidation are believed to be controlled through both primary and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomass
Biomass is plant-based material used as a fuel for heat or electricity production. It can be in the form of wood, wood residues, energy crops, agricultural residues, and waste from industry, farms, and households. Some people use the terms biomass and biofuel interchangeably, while others consider biofuel to be a ''liquid'' or ''gaseous'' fuel used for transportation, as defined by government authorities in the US and EU. The European Union's Joint Research Centre defines solid biofuel as raw or processed organic matter of biological origin used for energy, such as firewood, wood chips, and wood pellets. In 2019, biomass was used to produce 57 EJ (exajoules) of energy, compared to 190 EJ from crude oil, 168 EJ from coal, 144 EJ from natural gas, 30 EJ from nuclear, 15 EJ from hydro and 13 EJ from wind, solar and geothermal combined. Approximately 86% of modern bioenergy is used for heating applications, with 9% used for transport and 5% for electricity. Most of the global b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer ( /èˆùnûÎnti.èmèr, è-, -oò-/ ''ih-NAN-tee-è-mèr''; from Ancient Greek Ã¥ö§ö˜ö§üö¿ö¢ü ''(enûÀntios)'' 'opposite', and ö¥öÙüö¢ü ''(mûˋros)'' 'part') ã also called optical isomer, antipode, or optical antipode ã is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |