|
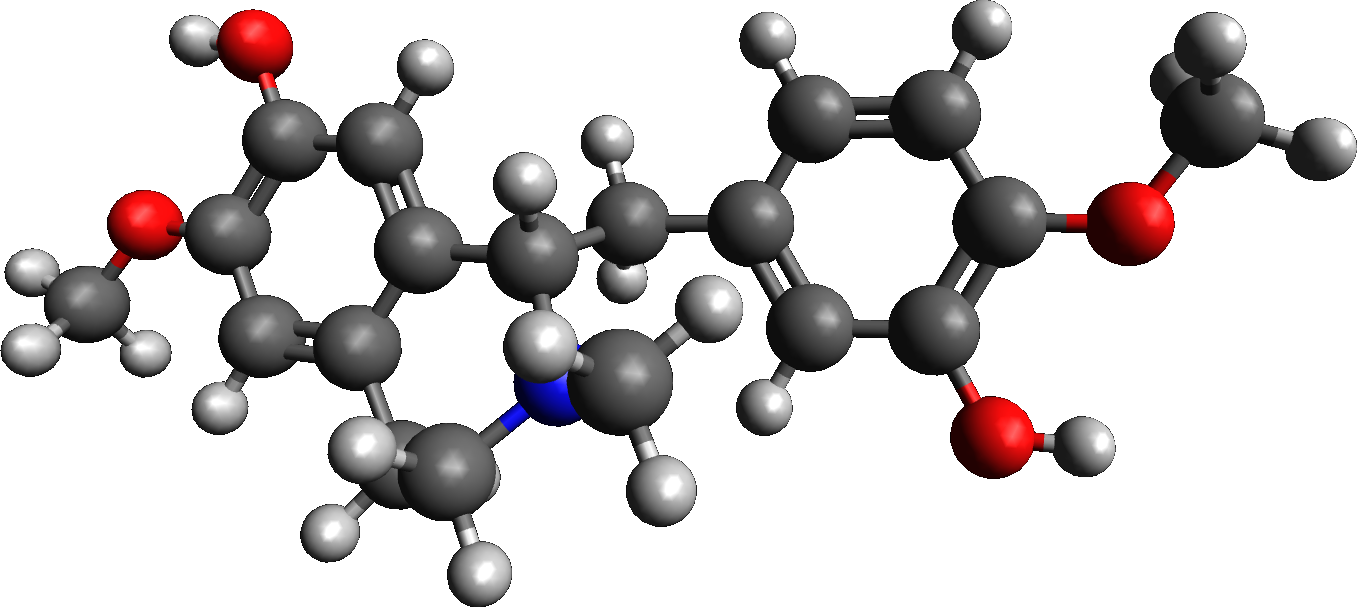
(R)-reticuline
Reticuline is a chemical compound found in a variety of plants including ''Lindera aggregata'', '' Annona squamosa'', and '' Ocotea fasciculata'' (also known as ''Ocotea duckei''). It is based on the benzylisoquinoline structure. Reticuline is one of the alkaloids found in opium, and experiments in rodents suggest it possesses potent central nervous system depressing effects. It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of atypical parkinsonism known as Guadeloupean Parkinsonism. Metabolism 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses ''S''-adenosyl methionine and 3'-hydroxy-''N''-methyl-(''S'')-coclaurine to produce ''S''-adenosylhomocysteine and (''S'')-reticuline. Reticuline oxidase uses (''S'')-reticuline and O2 to produce (''S'')-scoulerine and H2O2. Salutaridine synthase uses (''R'')-reticuline, NADPH, H+, and O2 to produce salutaridine, NADP+, and H2O. Salutaridine can then be tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salutaridine Synthase
In enzymology, a salutaridine synthase () is an enzyme that catalyzes the chemical reaction :(R)-reticuline + NADPH + H+ + O2 \rightleftharpoons salutaridine + NADP+ + 2 H2O The 4 substrates of this enzyme are (R)-reticuline, NADPH, H+, and O2, whereas its 3 products are salutaridine, NADP+, and H2O. This enzyme belongs to the family of oxidoreductases, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with NADH or NADPH as one donor, and the other dehydrogenated. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivial ... of this enzyme class is (R)-reticuline,NADPH:oxygen oxidoreductase (C-C phenol-coupling). This enzyme is also called (R)-reticuline ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dehydroreticulinium Reductase (NADPH)
In enzymology, a 1,2-dehydroreticulinium reductase (NADPH) () is an enzyme that catalyzes the chemical reaction :(R)-reticuline + NADP \rightleftharpoons 1,2-dehydroreticulinium + NADPH + H Thus, the two substrates of this enzyme are (R)-reticuline and NADP, whereas its 3 products are 1,2-dehydroreticulinium, NADPH, and H. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-NH group of donors with NAD+ or NADP+ as acceptor. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivial ... of this enzyme class is (R)-reticuline:NADP+ oxidoreductase. This enzyme is also called 1,2-dehydroreticulinium ion reductase. This enzyme participates in alkaloid biosynthesis i. References * {{Portal bar, Biology, border=no EC 1.5.1 N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticuline
Reticuline is a chemical compound found in a variety of plants including ''Lindera aggregata'', ''Annona squamosa'', and '' Ocotea fasciculata'' (also known as ''Ocotea duckei''). It is based on the benzylisoquinoline structure. Reticuline is one of the alkaloids found in opium, and experiments in rodents suggest it possesses potent central nervous system depressing effects. It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of atypical parkinsonism known as Guadeloupean Parkinsonism. Metabolism 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses ''S''-adenosyl methionine and 3'-hydroxy-''N''-methyl-(''S'')- coclaurine to produce ''S''-adenosylhomocysteine and (''S'')-reticuline. Reticuline oxidase uses (''S'')-reticuline and O2 to produce (''S'')-scoulerine and H2O2. Salutaridine synthase uses (''R'')-reticuline, NADPH, H+, and O2 to produce salutaridine, NADP+, and H2O. Salutaridine can then be t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coclaurine
Coclaurine is a nicotinic acetylcholine receptor antagonist which has been isolated from a variety of plant sources including ''Nelumbo nucifera'', '' Sarcopetalum harveyanum'', '' Ocotea duckei'', and others. It belongs to the class of tetrahydroisoquinoline alkaloid Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...s. Dimerization of coclaurine leads to the biscoclaurine alkaloids such as cepharanthine. References Nicotinic antagonists Benzylisoquinoline alkaloids Phenols {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Opium Alkaloids
Nature, in the broadest sense, is the physics, physical world or universe. "Nature" can refer to the phenomenon, phenomena of the physical world, and also to life in general. The study of nature is a large, if not the only, part of science. Although humans are part of nature, human activity is often understood as a separate category from other natural phenomena. The word ''nature'' is borrowed from the Old French ''nature'' and is derived from the Latin word ''natura'', or "essential qualities, innate disposition", and in ancient times, literally meant "birth". In ancient philosophy, ''natura'' is mostly used as the Latin translation of the Greek word ''physis'' (φύσις), which originally related to the intrinsic characteristics of plants, animals, and other features of the world to develop of their own accord. The concept of nature as a whole, the physical universe, is one of several expansions of the original notion; it began with certain core applications of the word � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dehydroreticulinium
Onekama ( ) is a village in Manistee County, Michigan, Manistee County in the U.S. state of Michigan. The population was 411 at the 2010 United States Census, 2010 census. The village is located on the shores of Portage Lake (Michigan), Portage Lake and is surrounded by Onekama Township, Michigan, Onekama Township. The town's name is derived from "Ona-ga-maa," an Anishinaabe word which means "singing water." Geography According to the United States Census Bureau, the village has a total area of , all land. The M-22 highway runs through downtown Onekama. History The predecessor of the village of Onekama was the settlement of Portage at Portage Point, first established in 1845, at the western end of Portage Lake (Michigan), Portage, at the outlet of Portage Creek. In 1871, when landowners around the land-locked lake became exasperated with the practices of the Portage Sawmill, they took the solution into their own hands and dug a channel through the narrow isthmus, opening a waterw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oripavine
Oripavine is an opioid and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions. Pharmacological properties Oripavine possesses an analgesic potency comparable to morphine; however, it is not clinically useful due to severe toxicity and low therapeutic index. In both mice and rats, toxic doses caused tonic-clonic seizures followed by death, similar to thebaine. Oripavine has a potential for dependence which is significantly greater than that of thebaine but slightly less than that of morphine. Bridged derivatives Of much greater relevance are the properties of the orvinols, a large family of semi-synthetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thebaine
Thebaine (paramorphine), also known as codeine methyl enol ether, is an opiate alkaloid, its name coming from the Greek Θῆβαι, '' Thēbai'' (Thebes), an ancient city in Upper Egypt. A minor constituent of opium, thebaine is chemically similar to both morphine and codeine, but has stimulatory rather than depressant effects. At high doses, it causes convulsions similar to strychnine poisoning. The synthetic enantiomer (+)-thebaine does show analgesic effects apparently mediated through opioid receptors, unlike the inactive natural enantiomer (−)-thebaine. While thebaine is not used therapeutically, it is the main alkaloid extracted from ''Papaver bracteatum'' (Iranian opium / Persian poppy) and can be converted industrially into a variety of compounds, including hydrocodone, hydromorphone, oxycodone, oxymorphone, nalbuphine, naloxone, naltrexone, buprenorphine, butorphanol and etorphine. Thebaine is controlled under international law, is listed as a Class A drug under the Mis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salutaridine
Salutaridine, also known as floripavine, is an alkaloid that is present in the morphinian alkaloid pathway of opium poppy. Its biosynthetic precursor is the alkaloid reticuline, (''R'')-reticuline. (''R'')-Reticuline is converted to salutaridine by the enzyme salutaridine synthase. Salutaridine is converted to salutaridinol by the enzyme salutaridine reductase (SalR), with the reduction of NADPH to NADP+. References Morphinans Phenols Enones Phenol ethers {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scoulerine
Scoulerine, also known as discretamine and aequaline, is a benzylisoquinoline alkaloid (BIA) that is derived directly from (''S'')-reticuline through the action of berberine bridge enzyme. It is a precursor of other BIAs, notably berberine, noscapine, (''S'')- tetrahydropalmatine, and (''S'')-stylopine, as well as the alkaloids protopine, and sanguinarine. It is found in many plants, including opium poppy, ''Croton flavens'', and certain plants in the genus '' Erythrina''. Studies show that scoulerine is an antagonist ''in vitro'' at the α2-adrenoceptor, α1D-adrenoceptor and 5-HT receptor 5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory ne .... It has also been found to be a GABAA receptor agonist ''in vitro''. References {{reflist Isoquinolinoisoquinolines Natural opium al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticuline Oxidase
In enzymology, a reticuline oxidase () is an enzyme that catalyzes the chemical reaction :(S)-reticuline + O2 \rightleftharpoons (S)-scoulerine + H2O2 Thus, the two substrates of this enzyme are (S)-reticuline and O2, whereas its two products are (S)- scoulerine and H2O2. This enzyme belongs to the family of oxidoreductases, specifically those acting on X-H and Y-H to form an X-Y bond with oxygen as acceptor. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivial ... of this enzyme class is (S)-reticuline:oxygen oxidoreductase (methylene-bridge-forming). Other names in common use include BBE, berberine bridge enzyme, berberine-bridge-forming enzyme, and tetrahydroprotoberberine synthase. This enzyme participates in alkaloid biosynthesis i. References * * * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)