|
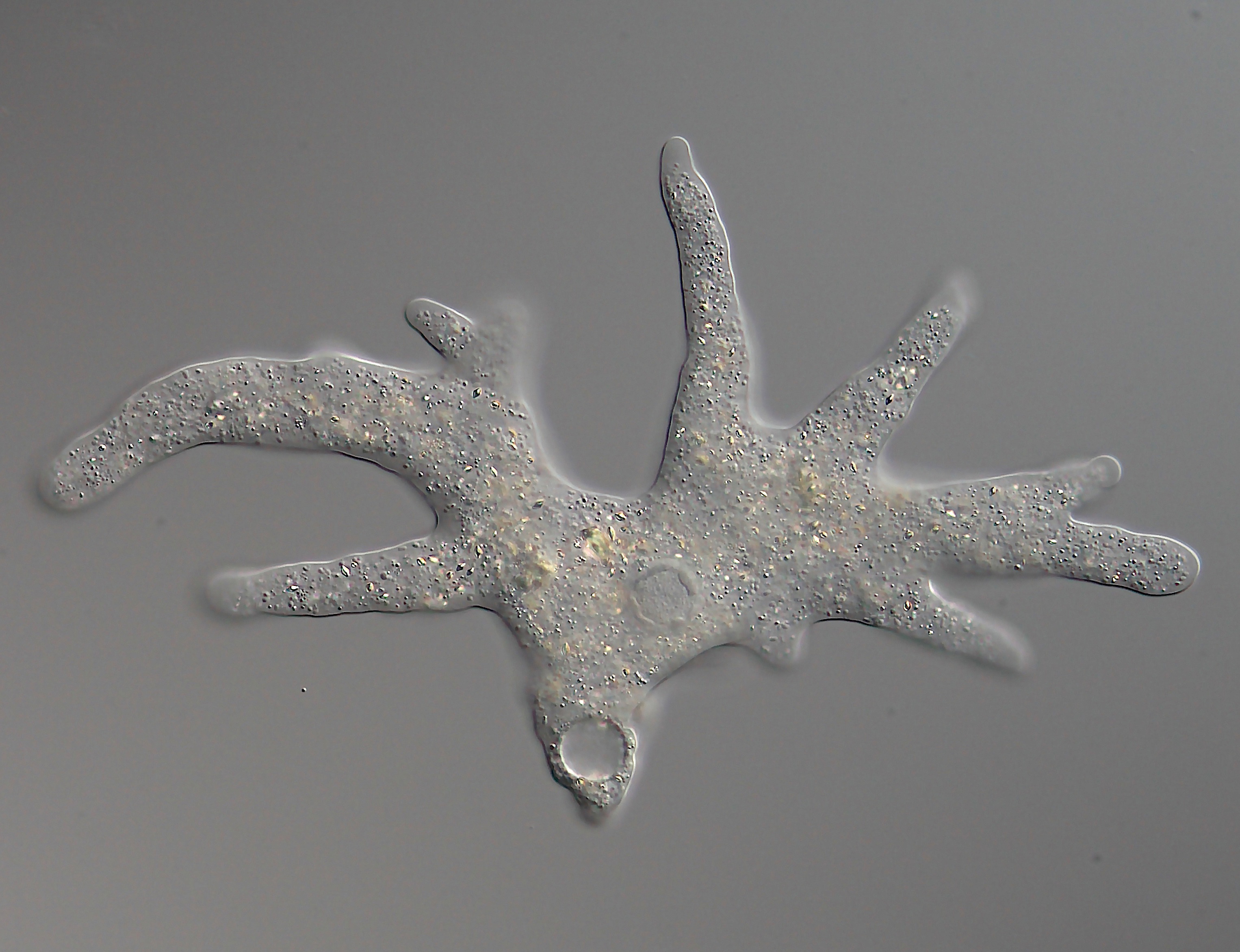
Filopodia
Filopodia (singular filopodium) are slender cytoplasmic projections that extend beyond the leading edge of lamellipodia in migrating cells. Within the lamellipodium, actin ribs are known as ''microspikes'', and when they extend beyond the lamellipodia, they're known as filopodia. They contain microfilaments (also called actin filaments) cross-linked into bundles by actin-bundling proteins, such as fascin and fimbrin. Filopodia form focal adhesions with the substratum, linking them to the cell surface. Many types of migrating cells display filopodia, which are thought to be involved in both sensation of chemotropic cues, and resulting changes in directed locomotion. Activation of the Rho family of GTPases, particularly cdc42 and their downstream intermediates, results in the polymerization of actin fibers by Ena/Vasp homology proteins. Growth factors bind to receptor tyrosine kinases resulting in the polymerization of actin filaments, which, when cross-linked, make up the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filopodia
Filopodia (singular filopodium) are slender cytoplasmic projections that extend beyond the leading edge of lamellipodia in migrating cells. Within the lamellipodium, actin ribs are known as ''microspikes'', and when they extend beyond the lamellipodia, they're known as filopodia. They contain microfilaments (also called actin filaments) cross-linked into bundles by actin-bundling proteins, such as fascin and fimbrin. Filopodia form focal adhesions with the substratum, linking them to the cell surface. Many types of migrating cells display filopodia, which are thought to be involved in both sensation of chemotropic cues, and resulting changes in directed locomotion. Activation of the Rho family of GTPases, particularly cdc42 and their downstream intermediates, results in the polymerization of actin fibers by Ena/Vasp homology proteins. Growth factors bind to receptor tyrosine kinases resulting in the polymerization of actin filaments, which, when cross-linked, make up the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
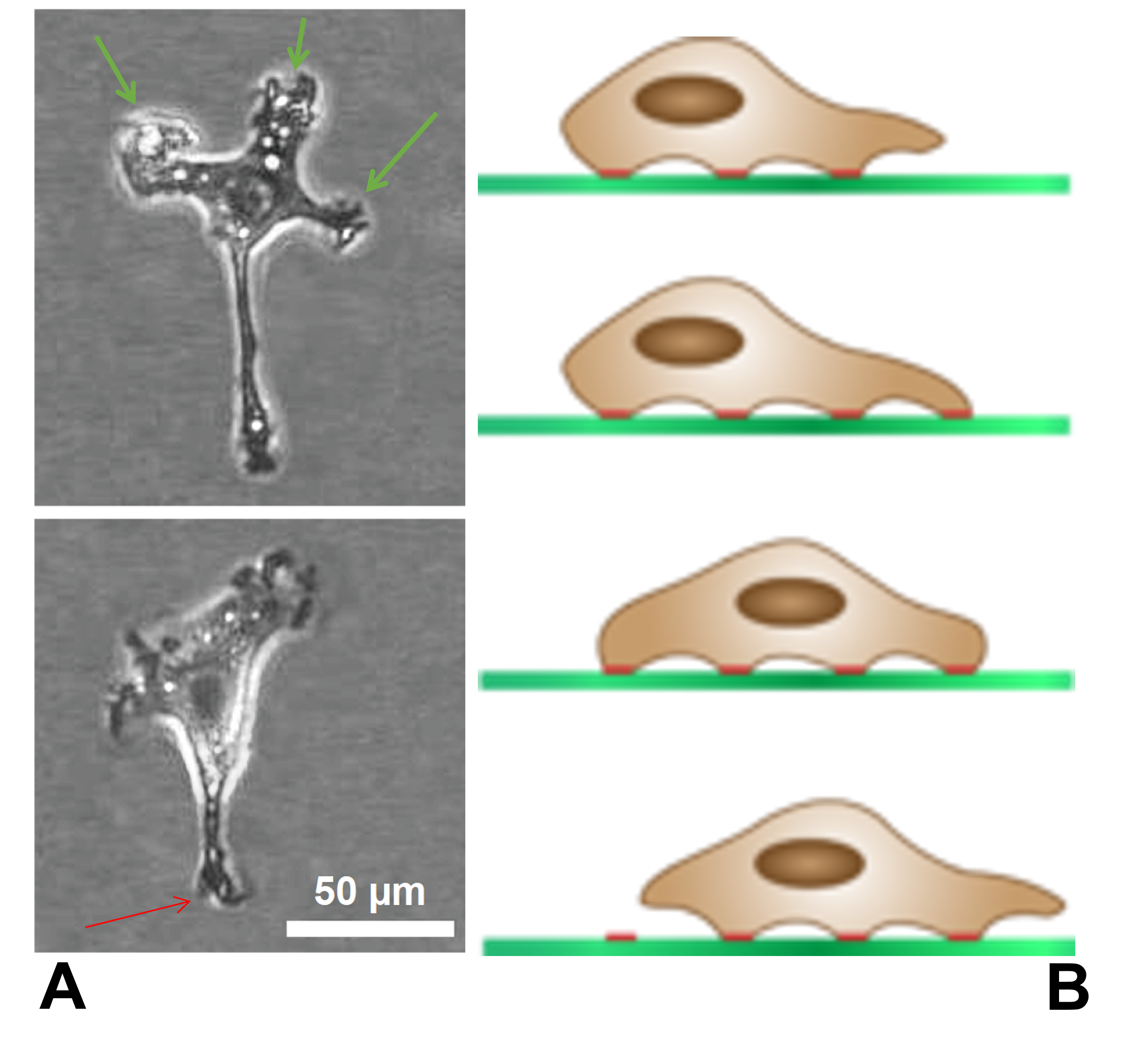
Growth Cone
A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth. Their existence was originally proposed by Spanish histologist Santiago Ramón y Cajal based upon stationary images he observed under the microscope. He first described the growth cone based on fixed cells as "a concentration of protoplasm of conical form, endowed with amoeboid movements" (Cajal, 1890). Growth cones are situated on the tips of neurites, either dendrites or axons, of the nerve cell. The sensory, motor, integrative, and adaptive functions of growing axons and dendrites are all contained within this specialized structure. Structure The morphology of the growth cone can be easily described by using the hand as an analogy. The fine extensions of the growth cone are pointed filopodia known as microspikes. The filopodia are like the "fingers" of the growth cone; they contain bundles of actin filaments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rho Family Of GTPases
The Rho family of GTPases is a family of small (~21 kDa) signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions. History Identification of the Rho family of GTPases began in the mid-1980s. The first identified Rho member was RhoA, isolated serendipitously in 1985 from a low stringency cDNA screening. Rac1 and Rac2 were identified next, in 1989 followed by Cdc42 in 1990. Eight additional mammalian Rho members were identified from biological screenings until the late 1990s, a turning point in biology where availability of complete genome seq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fimbrin
Fimbrin also known as is plastin 1 is a protein that in humans is encoded by the PLS1 gene. Fimbrin is an actin cross-linking protein important in the formation of filopodia. Structure Fimbrin belongs to the calponin homology (CH) domain superfamily of actin cross-linking proteins. Like other members of this superfamily, which include α-actinin, β- spectrin, dystrophin, ABP-120 and filamin, it has a conserved 27 kDa actin-binding domain that contains a tandem duplication of a sequence that is homologous to calponin. In addition to cross-linking actin filaments into bundles and networks, CH domains also bind intermediate filaments and some signal transduction proteins to the actin cytoskeleton. Structural comparison of actin filaments and fimbrin CH domain-decorated actin filaments has revealed changes in the actin structure due to fimbrin-mediated cross-linking that may affect the actin filaments' affinity for other actin-binding proteins and may be part of the regulation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudopodia
A pseudopod or pseudopodium (plural: pseudopods or pseudopodia) is a temporary arm-like projection of a eukaryotic cell membrane that is emerged in the direction of movement. Filled with cytoplasm, pseudopodia primarily consist of actin filaments and may also contain microtubules and intermediate filaments. Pseudopods are used for motility and ingestion. They are often found in amoebas. Different types of pseudopodia can be classified by their distinct appearances. Lamellipodia are broad and thin. Filopodia are slender, thread-like, and are supported largely by microfilaments. Lobopodia are bulbous and amoebic. Reticulopodia are complex structures bearing individual pseudopodia which form irregular nets. Axopodia are the phagocytosis type with long, thin pseudopods supported by complex microtubule arrays enveloped with cytoplasm; they respond rapidly to physical contact. Some pseudopodial cells are able to use multiple types of pseudopodia depending on the situation: Most of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamellipodia
The lamellipodium (plural lamellipodia) (from Latin ''lamella'', related to ', "thin sheet", and the Greek radical ''pod-'', "foot") is a cytoskeletal protein actin projection on the leading edge of the cell. It contains a quasi-two-dimensional actin mesh; the whole structure propels the cell across a substrate. Within the lamellipodia are ribs of actin called microspikes, which, when they spread beyond the lamellipodium frontier, are called filopodia. The lamellipodium is born of actin nucleation in the plasma membrane of the cell and is the primary area of actin incorporation or microfilament formation of the cell. Description Lamellipodia are found primarily in all mobile cells, such as the keratinocytes of fish and frogs, which are involved in the quick repair of wounds. The lamellipodia of these keratinocytes allow them to move at speeds of 10–20 μm / min over epithelial surfaces. When separated from the main part of a cell, a lamellipodium can stil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microfilament
Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fascin
Fascin is an actin bundling protein. Species and tissue distribution It is a 54-58 kilodalton monomeric actin filament bundling protein originally isolated from sea urchin egg but also found in ''Drosophila'' and vertebrates, including humans. Fascin (from the Latin for ''bundle'') is spaced at 11 nanometre intervals along the filament. The bundles in cross section are seen to be hexagonally packed, and the longitudinal spacing is compatible with a model where fascin cross-links at alternating 4 and 5 actins. It is calcium insensitive and monomeric. Three forms of fascin are found in vertebrates: Fascin1, widely found in the nervous system and elsewhere; fascin2 found in the retinal photoreceptor cells; fascin3, which is only found in the testes. Function Fascin binds beta-catenin, and colocalizes with it at the leading edges and borders of epithelial and endothelial cells. The role of Fascin in regulating cytoskeletal structures for the maintenance of cell adh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ena/Vasp Homology Proteins
ENA/VASP homology proteins or EVH proteins are a family of closely related proteins involved in cell motility in vertebrate and invertebrate animals. EVH proteins are modular proteins that are involved in actin polymerization, as well as interactions with other proteins. Within the cell, Ena/VASP proteins are found at the leading edge of Lamellipodia and at the tips of filopodia. Ena, the founding member of the family was discovered in a drosophila genetic screen for mutations that act as dominant suppressors of the abl non receptor tyrosine kinase. Invertebrate animals have one Ena homologue, whereas mammals have three, named Mena, VASP, and Evl. Ena/VASP proteins promote the spatially regulated actin polymerization required for efficient chemotaxis in response to attractive and repulsive guidance cues. Mice lacking functional copies of all three family members display pleiotropic phenotypes including exencephaly, edema, failures in neurite formation, and embryonic lethality ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin Filaments
Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-depen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the mobility and contraction of cells during cell division. Actin participates in many important cellular processes, including muscle contraction, cell motility, cell division and cytokinesis, vesicle and organelle movement, cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ehrlichia
''Ehrlichia'' is a genus of Rickettsiales bacteria that are transmitted to vertebrates by ticks. These bacteria cause the disease ehrlichiosis, which is considered zoonotic, because the main reservoirs for the disease are animals. ''Ehrlichia'' species are obligately intracellular pathogens and are transported between cells through the host cell filopodia during initial stages of infection, whereas in the final stages of infection, the pathogen ruptures the host cell membrane. History The genus ''Ehrlichia'' is named after German microbiologist Paul Ehrlich. The first ehrlichial disease was recognized in South Africa during the 19th century. Its tick-borne nature was determined in 1900. The organism itself was demonstrated in 1925 when it was recognized to be a ''Rickettsia''. It was initially named ''Rickettsia ruminantium'', and is currently named ''Ehrlichia ruminantium''. In 1945, an "infection and treatment" method for livestock was developed. This is still the only com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |